
Case Report
Ann Hematol Onco. 2023; 10(1): 1414.
Isolated Intra-Oral Relapsed B-Acute Lymphoblastic Leukemia (B-ALL): A Case Report
Al-Mohaya MA1, Al-Ali MA2, Binmayouf MS3, Sewaralthahab SS4* and Elyamany G5
1Consultant of Oral Medicine, Oral Medicine & Special Care Dentistry, Prince Sultan Military Medical City, KSA
2Resident of Saudi Board of Oral Medicine and Patholog, Prince Sultan Military Medical City, Riyadh, KSA
3Medical Intern, King Saud University, KSA
4Assistant Professor of Internal Medicine & Consultant Hematologist, King Saud University College of Medicine, King Saud University Medical City, Saudi Arabia.
5Consultant of Hematopathology, Department of Central Military Laboratory and Blood Bank, Saudi Arabia
*Corresponding author: Sarah Salah Sewaralthahab Assistant Professor of Internal Medicine & Consultant Hematologist, King Saud University College of Medicine, King Saud University Medical City, Riyadh, Saudi Arabia.
Received: December 15, 2022; Accepted: January 13, 2022; Published: January 20, 2023
Abstract
B-Lymphoblastic Leukemia (B-ALL) is the second most common acute leukemia in adults. Its clinical manifestations include leukocytosis or leukopenia, anemia, and thrombocytopenia with an overall poor prognosis in elderly patients. If relapse occurs, it commonly involves the bone marrow but can also involve Extramedullary (EM) sites such as the brain, testis, liver, spleen and lymph nodes. Isolated extramedullary involvement of the oral cavity in relapse B-ALL is extremely rare with only a few reposted cases in the literature in pediatrics and Adolescent-and-Young-Adult (AYA) population. Here we describe the case of an elderly gentleman who presented with isolated EM B-ALL relapse involving the oral cavity and gnathic bones which was initially misdiagnosed as an odontogenic infection.
Keywords: Relapse; Acute lymphoblastic leukemia; Lymphoblastic lymphoma; Oral cavity; Gnathic bone
Abbreviations: B-ALL: B-Lymphoblastic Leukemia; EM: Extramedullary; B-LBL: B-Lymphoblastic Lymphoma; AYA: Adolescent And Young Adults; FISH: Fluorescence In Situ Hybridization, CVD: Cyclophosphamide, Vincristine, Dexamethasone; POMP: Mercaptopurine, Vincristine, Methotrexate, Prednisone; HSCT: Hematopoietic Stem Cell Transplantation
Introduction
B-Lymphoblastic Leukemia (B-ALL) is a neoplasm of precursor lymphoid cells committed to the B cell lineage, involving the bone marrow, blood, and at times Extramedullary sites (EM) with a blast percentage of more than 20% [1]. It represents 80–85% of Acute Lymphoblastic Leukemia (ALL), in contrast to B-Lymphoblastic Lymphoma (B-LBL) which accounts only for 10% [2]. By age, B-ALL is the most common malignant neoplasm found in pediatrics/ Adolescent and Young Adults (AYA) population with about 60% of cases diagnosed in those younger than 20 years of age and the second most common acute leukemia in adults [3].
Patients commonly present with fatigue, bone pain, bleeding, infections, anorexia, or malaise [4]. The CNS, testes, liver, spleen, and lymph nodes are common sites for extramedullary disease; other less common reported sites include the ovaries, breast, and kidneys. However, involvement of the head and neck area is quite uncommon [5,6] and B-ALL relapse primarily involving the oral cavity and gnathic bone are extremely rare with only a few reported cases in the literaturein pediatrics/ AYA [7,8].
Herein, we describe a case of a 78-year-old Saudi gentleman with a history of B-ALL who presented with an intraoral swelling as an isolated manifestation of relapsed B-ALL.
Case Report
A 78 -year-old Saudi gentleman was diagnosed with B-ALL in June 2019 after presenting to the emergency department at Prince Sultan Medical Military City with fever, confusion, and severe generalized bony pain limiting his mobility. He had a medical history of chronic renal impairment, hypertension, dyslipidemia, and long-standing heavy smoking. At presentation, laboratory investigation showed elevations in creatinine (122 umol/L) and urea (7.7 mmol/L) leukopenia (0.7 x 10^9/L), anemia (hemoglobin 7.5 g/dL), thrombocytopenia (platelet count 47x 10^9/L) and low red blood cell count (2.7 x10^12/L). Further investigations revealed a normal free light chain ratio and normal immunoglobulin level. A plain radiograph was done showing multiple lytic lesions involving the pelvic bone. CT scan showed enlarged left para-aortic lymph node measuring 3.4x2.5 cm with no other lymphadenopathy and no hepatosplenomegaly.
Bone marrow aspiration and core biopsy showed a hypercellular marrow with normal trilineage hematopoiesis being replaced by infiltrating mononuclear malignant cells with round to oval, large, and slightly irregular nuclei having open chromatin, scant cytoplasm, and prominent nucleoli (blast cells, approximately 93% of BM cells). Flow cytometry analysis showed the blast cells express CD19, CD10, CD20 (dim),CD22, CD79a, HLADR and negative for MPO, CD3, CD34, TdT and other markers. On immunohistochemistry, the cells were positive for PAX 5, CD20 (weak), and BCL2 and negative for Kappa, lambda, MPO, CD3, TdT and CD34. Fluorescence in situ hybridization (FISH) analysis showed clonal mutations with MYC gene 8q24 rearrangement and duplications of the MLL 11q23 and ETV6-RUNX1 t (12:21) rearrangement.
Following diagnosis, the patient was placed on reducedintensity chemotherapy with mini-hyper-CVD (cyclophosphamide, vincristine, dexamethasone - part A included 50% reduction of cyclophosphamide and dexamethasone plus 2 mg of vincristine and no anthracycline, while part B included methotrexate 250mg/m2 and 4 doses of cytarabine 0.5 mg/m2). His treatment course was complicated by repeated attacks of severe neutropenic sepsis requiringmultiple hospitalizations. Maintenance chemotherapywith POMP (Mercaptopurine, Vincristine, Methotrexate, Prednisone) was also modified and reduced according to the patient’s tolerance as well. Follow-up bone marrow examination showed achievement of morphological remission with normal trilineage hematopoiesis. FISH confirmed resolution of chromosomal abnormalities previously detected at diagnosis.
In December 2020the patient presented to the oral medicine clinic with palatal swelling that fluctuated in size and responded minimally after empiric antibiotics and extraction of unsalvageable teeth. Extraoral examination was unremarkable with no cervical lymphadenopathy. However, the intraoral examination revealed a sessile dome-shaped exophytic palatal mass that extended to the alveolar ridge of extracted teeth #11 and #21. It had a smooth surface and was pinkish to purple in color with focal red discoloration where the lower teeth came in contact with the mass. The mass was fibrous in its consistency and measured about 3x5 cm in size (Figure 1). A CT scan revealed an asymmetrical destructive mass and MRI showed an enhanced soft tissue mass involving the anterior paramedian aspect of the maxilla extending to involve the alveolar process and floor of the nasal cavity.Laboratory investigation revealed elevations in creatinine level (125umol/L) and urea (6.4 mmol/L). White blood cell count was3.1x 10^9/L, platelet count was218 10^9/L and hemoglobin level was 10.2 g/dL. Peripheral blood smear showed no blast cells. Bone marrow aspiration and core biopsy showed hypocellular marrow with no increase in blast cells. An incisional biopsy from the palatal mass was performed under local anesthesia with histopathologic examination revealing diffuse infiltration of small to medium size oval to round cells with open chromatin and scanty cytoplasm. The tumor cells were positive for CD10, CD45, CD20 (week and subset), TdT (focal), and BCL-2 with high Ki-67 proliferation rate (70%-80%), but negative to CD3, CD30, and CD34 (Figure 2).Morphological and immunohistochemical profiles were consistent with extramedullary relapse of B-ALL.
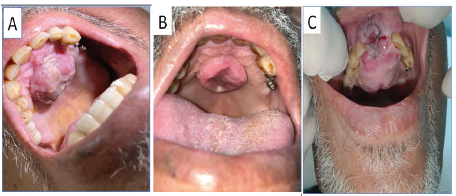
Figure 1: Clinical presentation of the lesion in the oral cavity: (A)
Intraoral image of the lesion at the initial visit appearing as nodular
dome- shaped exophytic palatal mass. (B) Intraoral image showing
regression of the lesion after 7 days of empiric antibiotic usage. (C)
Intraoral photograph at the time of follow up visit showing pinkish
to purple sessile dome-shaped exophytic palatal mass that extended
to the alveolar ridge of extracted teeth #11,21.
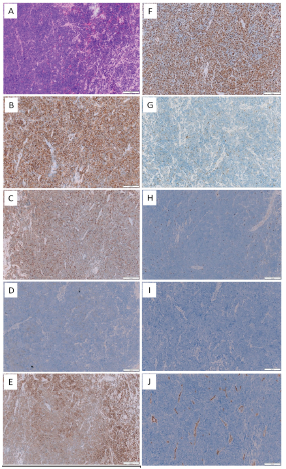
Figure 2: Microscopical and immunohistochemical findings: (A) The
histopathological examination revealed an exuberant lymphoid
proliferation containing small to medium sized oval to round cells
with open chromatin and scanty cytoplasm (H & E, x20). High power
views of immunohistochemical stain B-J. The cells are (B) CD45
positive. (C) CD10 positive. (D) Week and subset CD20 positivity. (E)
BCL-2 positive. (F) High Ki67 expression. (G) TDT focally positive. (H)
CD3 negative. (I) CD30 negative. (J) CD34 negative.
Given the patient’s functional status, comorbidities and lack of bone marrow involvement, he was treated with low-dose radiotherapy as palliation therapy. Unfortunately, the patient passed away in April 2021 secondary to septic shock.
Discussion
Despite the high rate of response of B-ALL to induction chemotherapy and the advances in therapeutic options, relapse still occurs [9]. Reporting of EM relapse of ALL has recently increased, especially with the advent of Hematopoietic Stem Cell Transplantation (HSCT). Relapse can occur in isolation or with bone marrow involvement. Most of the literatureon relapse is noted in pediatric patients given the predilection of the disease to occur in the pediatric/AYA population and the limited information regarding EM relapse in adults [5,8,10-12]. Overall, the prognosis of ALL in the elderly patient is poor, in contrast to the pediatric patients who have the benefit of less comorbidities and ability to withstand intensive therapy with or without HSCT both initially. The timing of EM relapse (early:<18 months from diagnosis, late:= 18 months from diagnosis) carries a prognostic significance with better outcomes noted in later relapses without bone marrow involvement [12]. Very late relapses (≥5 years from initial diagnosis) are less frequently and typically seen in adults [13]. The locations of EM relapse include the skin/soft tissue, brain, testis and lymph nodes; extramedullary relapse involving the oral cavity is rare (10). On review of literature, only four other cases of EM relapse of B-ALL affecting the oral cavity and the gnathic bone were found. Clinical presentation varied from painless swelling to sensory neural changes; treatment choices and outcomes were only available in some of the cases (Table 1).
Authors
Age
Gender
Timing of Relapse
Site
Clinical presentation
Radiographic presentation
PB/BM involvement
IHC
Genetic
Treatment
Follow up
Our case
78
M
Less than 18months
Maxilla
Asymptomatic palatal swelling extended to the alveolar ridge of extracted teeth #11,21
History of
B-ALLCT & MRI showed an asymmetrical destructive mass involving the anterior paramedian aspect of the maxilla which extended to the alveolar process and the floor of the nasal cavity
No
positive for CD45, CD20
(Week and subset) CD10, TdT (focal), and BCL-2 with high Ki-67 (70%-80%), and negative to MPO, CD3, CD30, CD15 and CD34Bone marrow was positive to MYC gene 8q24 rearrangement and duplications of the MLL 11q23 and ETV6-RUNX1 t (12:21) rearrangement at the time of initial diagnosis with B-ALL
Palliative radiotherapy
Died after
[15]
22
M
>5 years
Mandible
diminished sensation
over the mental area, and sialorrhea
diminished sensation
over the mental area, and sialorrhea
diminished sensation over the metal area and sialorrhea.History of
B-ALLMRI T 2 signal shows hyperintensity within the body and ramus of the left mandible and with less degree within the right mandible.
yes
NM
No
Chemotherapy and transplant
Alive
[8]
19
F
10 months after HSCT
Mandible
Swelling in the right infraorbital region, and left side of the mandible. paresthesia of right infraorbital nerve and anesthesia of left mental nerve Palpable bilateral submandibular LN. grade III teeth mobility.
History of
B-ALLOPG showed areas of
ill-defined radiolucency in the maxillary and mandibular
alveolar bone areas (apical to
teeth numbers 5, 6, 19, and 20) and the disappearance of the
inferior dental canal lines bilaterally.yes
Positive to CD10, CD79a and TdT
No
NM
NM
[10]
22
F
16 months
gingiva
rapid growing left lower gingival swelling.
History of
B-ALL.No
No
Positive to CD10,
CD19, CD22,
CD34, TdT and CD43No
NM
Died
[11]
10
F
3 years
Mandible
pain, localized to the alveolar ridge overlying the unerupted lower right second permanent molar.
History of B-ALL.
The dental radiograph showed absence of the cortical line and displaced wisdom tooth and unclear cortical outline of the right inferior dental canal and lower right third molar was displaced distally and occlusally
yes
Positive CD20, CD10, TdT, CD24, negative AE1/AE3, CD3, desmin
,myogenin, NSE, CD4, S100, CD99No
Chemotherapy and transplantation
Alive
Table 1: Clinicopathological and immunohistochemical features of relapsed B-ALL involving the oral cavity and gnathic bones previously published in the literature including our case.
This case describes an unusual presentation of isolated oral cavity EM relapse B-ALL in an elderly male. His initial work up at diagnosis was positive for ETV6-RUNX1, t(12;21) rearrangement, KMT2A gene 11q23 rearrangement, and MYC gene rearrangement. The ETV6-RUNX1 fusion is the most common genetic abnormality in pediatric B-ALL (about 25%) and less common in adult B-ALL (about 3%). It has a favorable prognosis in children but its effect on prognosis is undetermined in adults. The KMT2A gene rearrangement is found in 10% and 5% of adult and children ALL cases, respectively, with a bimodal incidence peaking in infants in the first 2 years of life then declining in the pediatric/AYA group before rising again in adults [14]. Over expression of the MYC gene may be associated with persistent disease and increased risk of relapse in a patient with B-ALL at any age [15].
Given the rarity of this presentation in comparison to the high rates of odontogenic abscesses in the oral cavity, his initial management as an infectious oral lesion was understandable. Dentists, oral medicine specialists, and oral surgeons play a key role in the early detection of such lesions. A suspicious lesion that does not respond to dental treatment and antibiotics should raise concerns with the treating physician. Thorough and careful examination of the oral cavity with a detailed medical history and proper investigations should be performed to reach a correct diagnosis; radiographic changes may aid in early detection and the correct diagnosis but ultimately definitive diagnosis requires histopathologic examination [9]. Delays indiagnosis in such cases result in delays treatment and worse prognosis.
Conclusion
In conclusion, extramedullary relapse of B-ALL in the oral cavity is extremely rare and may mimicodontogenic infections. This often leads to delays in diagnosis and treatment. Outcomes vary by age, bone marrow involvement and timing of relapse. Dentists, oral medicine, oral surgery specialists and hematologistsshould be well aware of the possibility of B-ALL relapse in the oral cavity and gnathic bone as they play a vital role in early detection.
References
- Borowitz MJ, Chan JKC, Downing JR, Le Beau MM, Arber DA. B lymphoblastic leukemia/lymphoma, not otherwise specified (NOS). In: WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2017; 200–2.
- Ries L, Kosary C, Hankey B, Miller B, Edwards B. SEER cancer statistics review, 1973–1996, Bethesda. MD Natl Cancer Inst. 1999.
- Brown P, Inaba H, Annesley C, Beck J, Colace S, Dallas M, et al. Pediatric acute lymphoblastic leukemia, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020; 18: 81–112.
- Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017; 7: e577.
- Skeith L, Lazo-Langner A, Mangel J. Kidney and pancreatic extramedullary relapse in adult acute lymphoblastic leukemia: a case report and review of the literature. Case Rep Hematol. 2013; 2013: 637264.
- Sánchez-Romero C, Pontes HAR, Pontes FSC, Rocha AC, Carlos R, Rendón JC, et al. Acute lymphoblastic leukemia/lymphoma of the oral and maxillofacial region. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018; 126: 152–64.
- Talreja KL, Barpande SR, Bhavthankar JD, Mandale MS. Precursor B-cell lymphoblastic lymphoma of oral cavity: A case report with its diagnostic workup. J Oral Maxillofac Pathol JOMFP. 2016; 20: 133–6.
- Bakathir AA, Al-Hamdani AS. Relapse of acute lymphoblastic leukemia in the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107: e14-16.
- Sava CN, Ritli L, Balmos AB, Iuhas AR, Marian P, Motorca MA, et al. Unusual extramedullary relapses in a case of common B-cell acute lymphoblastic leukemia. Case report and review of literature. Rom J Morphol Embryol. 2019; 60: 249–54.
- Au WY, Wong KY, Leung RYY, Tong ACK. Isolated gingival relapse of acute lymphoblastic leukemia after transplantation. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2008; 37: 249–51.
- Benson RE, Rodd HD, North S, Loescher AR, Farthing PM, Payne M. Leukaemic infiltration of the mandible in a young girl. Int J Paediatr Dent. 2007; 17: 145–50.
- Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008; 22: 2142–50.
- Ganzel C, Wang XV, Rowe JM, Richards SM, Buck G, Marks DI, et al. At three years, patients with acute lymphoblastic leukaemia are still at risk for relapse. Results of the international MRC UKALLXII/ECOG E2993 trial. Br J Haematol. 2020; 191: 37–43.
- Akkari YM, Bruyere H, Hagelstrom RT, Kanagal-Shamanna R, Liu J, Luo M, et al. Evidence-based review of genomic aberrations in B-lymphoblastic leukemia/lymphoma: Report from the cancer genomics consortium working group for lymphoblastic leukemia. Cancer Genet. 2020; 243: 52–72.
- Allen A, Gill K, Hoehn D, Sulis M, Bhagat G, Alobeid B. C-myc protein expression in B-cell acute lymphoblastic leukemia, prognostic significance? Leuk Res. 2014; 38: 1061–6.