
Case Report
Ann Hematol Onco. 2023; 10(1): 1416.
Jejunal Malignant Peripheral Nerve Sheath Tumor with Preoperative Suspicion of Gastrointestinal Stromal Tumor: Report of a Korean Case and Literature Review
Yoo KC1, Choe SY2, Byeon SJ3 and Kim JW4*
1Department of Surgery, Chungbuk National University, Chungbuk National University Hospital, Republic of Korea
2Hallym University College of Medicine, Chuncheon Si, Republic of Korea
3Department of pathology, Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Republic of Korea
4Department of Surgery, Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Republic of Korea
*Corresponding author: Kim jwJong Wan Kim, Department of Surgery, Dongtan Sacred Heart Hospital, Hallym University College of Medicine 40, Sukwoo-Dong, Hwaseong-Si, Gyeonggi-Do, Republic of Korea
Received: January 03, 2023; Accepted: February 17, 2023; Published: February 24, 2023
Abstract
Background: Malignant Peripheral Nerve Sheath Tumors (MPNSTs) are defined as malignant tumors arising from a peripheral nerve or displaying nerve sheath differentiation. We report the first Korean case of MPNST of the ileum.
Case Presentation: A 33-year-old female without family history of neurofibromatosis type I complained of lower abdominal pain. An abdomen computed tomography and enhanced magnetic resonance imaging of the pelvis confirmed a 5.6 cm heterogenous lobulated lesion in the ileum. Laparoscopic exploration revealed severe adhesion of the main ileal mass to the sigmoid colon. Laparoscopic adhesiolysis and segmental resection with anastomosis of the ileum was performed. On gross examination, the resected specimen was 6.5 cm and the tumor was located 10 cm proximal and 15 cm distal from the resection margins. In microscopic examination, the nucleus of the tumor cells was oval to elongated shape, showing moderate pleomorphism. Immunohistochemical staining showed that the main tumor cells were positive for S100 and about 10% of the tumor cells were positive for Ki-67. During the 12-month follow-up period, she presented no symptoms and the abdomen CT scans showed no evidence of distant metastasis in the thorax or abdominal recurrence.
Conclusion: We report a rare case of a young female with a MPNST of jejunum, suspected of having GIST before surgery and an extensive review of the literature for MPNST in intestinal tract.
Keywords: Malignant peripheral nerve sheath tumor; Jejunum
Background
Malignant Peripheral Nerve Sheath Tumors (MPNST) are malignant tumors that arise from a peripheral nerve or exhibit nerve sheath differentiation [1-3]. MPNSTs usually occur in the trunk, extremities, head, neck, or paravertebral regions [2]. MPNSTs are rare in the gastrointestinal tract and extremely rare in the bowel; only eight cases of MPNSTs in the small intestine [4-11] and five in the colon [12-16] have been reported in medical literature to date. Accordingly, the preoperative diagnosis, treatment, and prognosis of MPNSTs in the gastrointestinal tract are not well established. In this article, we describe the first reported Korean case of a MPNST in the jejunum and we compare this case with other reported cases.
Case Presentation
A 33-year-old female complaining of lower abdominal pain visited the Department of Obstetrics and Gynecology at a local hospital in March 2020. She reported suffering from intermittent chills and diarrhea 3 weeks before the onset of abdominal pain and vomiting. Abdominopelvic Computed Tomography (CT) images obtained at the local hospital revealed a 5.6 cm, heterogenous lobulated lesion in the distal ileum abutting the sigmoid colon and multiple slightly enlarged lymph nodes along the inferior mesenteric vein (Figure 1). She was then referred to the Department of Obstetrics and Gynecology at our hospital.
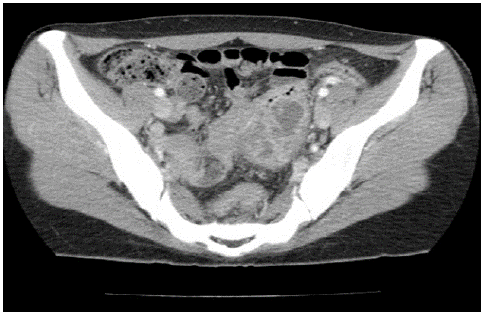
Figure 1: Computed tomography scan showing a 5.6 cm heterogeneous lobulated mass arising from the distal ileum.
The patient had previously given birth to two children by vaginal delivery. She had no family history of neurofibromatosis type 1, and unremarkable medical, surgical, and menstrual histories. Gynecological ultrasonography revealed a 6 cm heterogenous hyperechogenic mass in the left pelvic cavity with no abnormalities in the uterus or ovary. Enhanced Magnetic Resonance Imaging (MRI) of the pelvis confirmed a 5.6 cm heterogenous lobulated lesion in the ileum abutting a left ovarian vessel and a distal branch of the inferior mesenteric vessel in the left pelvic wall without abutting the sigmoid colon (Figure 2). The differential diagnoses were small bowel Gastrointestinal Stromal Tumor (GIST), endometriosis, and an inflammatory lesion of the pelvic cavity. The patient was referred to the Department of Surgery for further evaluation and surgery.
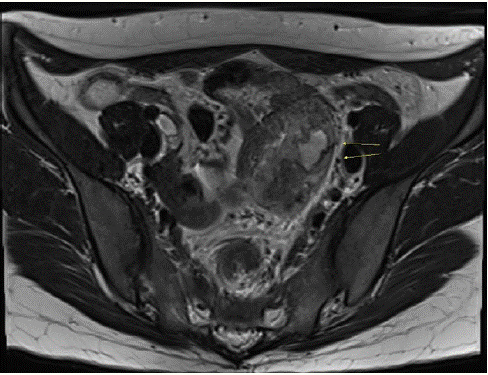
Figure 2: Magnetic resonance imaging scan showinga 5.6 cm heterogenous lobulated lesion in the ileum abutting a left ovarian vessel and a distal branch of the inferior mesenteric vessel in the left pelvic wall without abutting the sigmoid colon.
Laparoscopic exploration revealed severe adhesion of the main ileal mass to the sigmoid colon. After laparoscopic adhesiolysis, we extracted the small bowel mass through a 7 cm low-midline incision and performed resection and anastomosis of ileum. Furthermore, the enlarged lymph nodes were excised from the mesentery. Intraoperatively, the conglomerated lesion was suspected of invading the sigmoid colon, but this was later proven to be fibrous tissue by examination of frozen biopsy specimens.On gross examination, the resected specimen was 32.0 × 6.5 cm (Figure 3) and the tumor was located 10 cm proximal and 15 cm distal from the resection margins. The tumor dimensions were 6.0 × 4.5 × 4.0 cm. The tumor was white–gray in color and exhibited xanthogranulomatous inflammation.
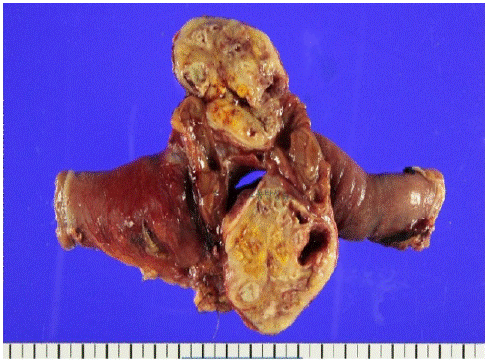
Figure 3: The tumor was white to grayish in color and showed a whirling pattern. A bright yellow color similar to xanthogranulomatous inflammation was observed throughout the tumor.
In microscopic examination, the nucleus of the tumor cells was oval to elongated shape, showing moderate pleomorphism. The cytoplasm is mainly elongated. Tumor cells showed organoid arrangement (Figure 4a). Immunohistochemical staining showed that the main tumor cells were positive for S100 and negative for CD117, Smooth Muscle Actin (SMA), and disFigure covered on GIST-1 (DOG1) (Figures 4b,4c). About 10% of the tumor cells were positive for Ki-67 (high proliferation index) (Figure 4d). Based on these findings, the tumor was compatible with a diagnosis of MPNST [16]. No evidence of metastasis was detected in seven dissected mesenteric lymph nodes.
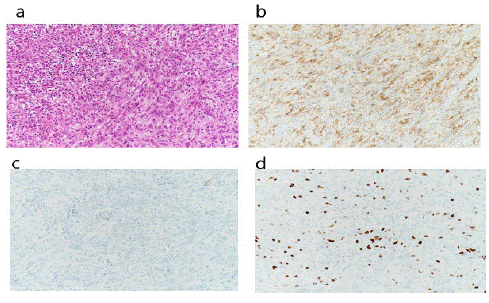
Figure 4: (a) Hematoxylin and eosin stain showing a necrotic area at the upper left and tumor cells at lower right (b) S-100 stain shows positive reaction (c) SMA stain shows negative reaction (d) KI-67 stain shows positive reaction.
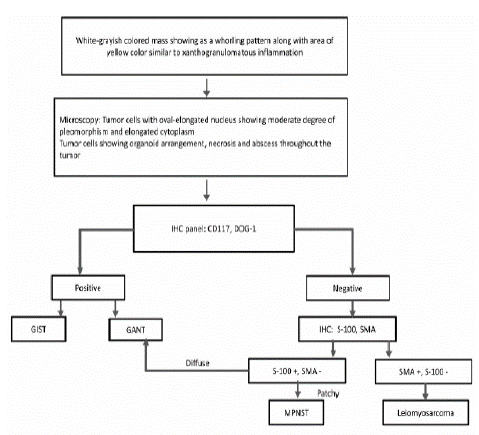
Figure 5: Diagnostic algorithm in Malignant peripheral nerve sheath tumors.
Six days after surgery, the patient was discharged with an uneventful course. At a regular follow-up 2 weeks later, her general condition was good. At 12 months, her chest and abdomen CT scans showed no evidence of distant metastasis in the thorax or abdominal recurrence.
Discussion
The World Health Organization defined MPNSTs as tumors originating from a peripheral nerve or displaying nerve sheath differentiation [1-3]. This term replaces the previously used terms malignant schwannoma, neurofibrosarcoma, and neurogenic sarcoma [1,2]. In adults, more than half of cases of MPNST are sporadic, other half are associated with neurofibromatosis type 1 [3,17]. The estimated prevalence of MPNST is 1.46 per 1,000,000 individuals [18].
Gastrointestinal MPNSTs are rare, especially in the small intestine and colon. A literature review from 1995 to the present revealed eight cases of MPNSTs in the small bowel [4-11] and five in the colon [12-16]. These cases are summarized in (Table 1 and 2).
The patients with MPNST in the small bowel ranged in age from 2 days to 71 years old [4-16]. Five of the patients were female and seven were male; the sex of one patient was not reported. The clinical symptoms of MPNST in the small bowel and colon were usually nonspecific, and included abdominal pain, nausea, vomiting, diarrhea, and weight loss. Among patients with MPNST of the small bowel, four [5,7,9,10] experienced obstruction symptoms due to ileal intussusceptions of the ileal mass and underwent emergency surgery. Our patient complained of lower abdominal pain preceded by chills and vomiting, which excluded MPNST in the small intestine from the preoperative list of differential diagnoses.
Preoperative diagnosis is often impossible due to the nonspecific and vague symptoms experienced by patients with MPNST. For preoperative diagnosis of MPNST in the small bowel, imaging techniques, such as MRI and contrast-enhanced CT [19], help to determine the tumor location, size, and local invasiveness to adjacent tissue. Quantitative fluorodeoxyglucose-positron emission tomography imaging can also help to differentiate benign PNST and MPNST based on the tumor’s metabolic activity [20]. Endoscopic biopsy is usually ineffective for preoperative diagnosis because the mucosa shows few abnormalities [21]. In our case, CT and MRI revealed a 5.6 cm heterogenous lobulated lesion in the distal ileum.
Pathological diagnosis of MPNSTs is also difficult and elusive due to the lack of standardized diagnostic criteria. In the small bowel, MPNSTs mostly grow from Schwann cells [22] and remain in the submucosa, but MPNSTs can grow exophytically or intraluminally [7]. On gross examination, MPNSTs cause fusiform-shaped nerve enlargement [22], like in our case, where the serosal mass had grown in traluminally and was pushing the mucosa away. Histopathologically, MPNSTs usually consist of spindle cells with a fascicular growth pattern on low-magnification images [22].
Although there are no specific immunohistochemical markers for MPNSTs, the focal reactivity of S-100 protein and electron microscopy can be helpful for the final diagnosis and differentiation [2,23]. S-100 is commonly used to identify neural-derived neoplasms but its immunoreactivity may not be present in all MPNSTs. If S-100 is present, the staining is usually focal and limited to a small number of cells [2]. Epithelioid MPNSTs typically show strong S-100 expression [22]. MPNSTs with better differentiation or lower grade also display stronger expression of S-100 than high-grade tumors [22]. MPNSTs often show high Ki-67 and p53 expression [24]. The differential diagnosis of MPNSTs includes GISTs, gastrointestinal autonomic nerve tumors, and a variety of sarcomas, like leiomyosarcoma [16]. Positivity for CD34 and CD117 and negativity for S-100 or desmin exclude GIST [16]. Meanwhile, negativity for CD34 and CD117, and positivity for SMA or desmin exclude leiomyosarcoma [16]. The tumor in our case was negative for CD117, SMA, and DOG1, and positive for S-100, inconsistent with possible diagnoses of GIST or leiomyosarcoma. Therefore, we diagnosed this tumor as a MPNST.
The optimal treatment of MPNSTs in the small intestine remains unknown because of their rarity [2]. Current treatment options are based on the approaches used to treat this type of tumors in other regions. Standard treatment of localized MPNSTs involves complete surgical resection with wide negative margins, which is also a strong predictor of survival [25]. In our case, we performed wide excision and confirmed clear resection margins and the absence of metastasis in the resected mesenteric lymph nodes.
Although various adjuvant therapies, such as chemotherapy, radiation therapy, target therapy and alcohol ablation have been suggested, the role of adjuvant therapy for MPNSTs is still controversial. Some studies have suggested that adjuvant radiotherapy is beneficial if clear resection margins are not possible [26]. However, radiation therapy is rarely beneficial for intestinal MPNSTs because of the location in the abdominal cavity [14]. Recently, some therapies targeting the molecular pathways of MPNST have been reported but further studies are required [23,25]. Ethanol injection also has been suggested in some studies. Chin et al reported four sessions of endoscopic ultrasound-guided alcohol ablation therapy for a small bowel MPNST in a patient whose general condition precluded surgery. The clinical response showed improvement in the patient’s diet and no residual tumor at the final endoscopy [8]. In our case, no signs of recurrence or metastasis were detected at the follow-ups. We decided to follow up our patient every 3 months and perform a CT evaluation every 6 months for early detection of any recurrence or distant metastases.
The prognosis of MPNSTs depends on various factors. The most significant prognostic factors for recurrence are tumor size (≥10 cm), tumor site, margin status, and histological grade [25,26]. The majority of MPNSTs are high-grade tumors with high risk of local recurrence and distant metastasis [2]. It has been reported that 40%–65% of cases of MPNST experience local recurrence and 30%–60% experience distant metastasis within 1 year of initial surgery [25]. Although the liver, bone, brain, and adrenal glands may be involved [25], the most common metastatic site is the lungs in two-thirds of cases.
The outcomes varied among the eight prior cases of MPNSTs of the small intestine (Table 1). Four patients had uneventful recurrence or metastasis [5,7-9]. One patient suffered from complications, including diarrhea, fever, and moderate anemia, without signs of tumor recurrence or metastasis, and died after the initial presentation [10]. Another patient suffered from as cites and peritoneal carcinomatosis and died before chemotherapy [6]. The other two patients suffered from multiple intra-abdominal tumors [4] and multiple metastases of the lung, bone, liver, spleen, and brain [11], respectively; both died 6 months after initial presentation. Our case showed no signs of progression of the resection margin and no metastasis at 12 months after surgery.
We report a rare case MPNST of jejunum presenting small bowel GIST before surgery. And, in a patient with a mesenchymal tumor, the possibility of MPNST should always be considered. Moreover, because there is no definitive guideline for treatment of small bowel MPNST, we recommend multidisciplinary approach for diagnosis and treatment of the tumors.
References
- Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986; 57: 2006-2021.
- Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors, 5th edn. Elsevier Health Sciences: St. Louis. 2007; 903–944.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114: 97-109.
- Nozu T, Takahashi A, Asakawa H, Uehara A, Kohogo Y, et al. Malignant Intestinal Schwannoma: A Case Report and a Review of the Literature in Japan. Intern Med. 1995; 34: 1101-1105.
- Telem DA, Pertsemlidis D. Malignant peripheral nerve sheath tumor: an unusual cause of intussusception. J Gastrointest Surg. 2008; 12: 1609-1611.
- Mohtaram A, Mesmoudi S, M’Rabti H, Rami A, Latib R, Bernoussi Z, et al. Malignant peripheral nerve sheath tumor of the small bowel: an unusual presentation with fatal outcome. Case Rep Oncol. 2013.
- Pandey D, Verma A, Akhtar A, ArsiaA, Singh N. Malignant Peripheral Nerve Sheath Tumour of Small Intestine Presenting as Ileo-Ileal Intussusception - A Rare Tumour with Unusual Complication. J ClinDiagn Res. 2015; 9(5):10503-10504.
- Chin M, Chen CL, Chang K, Lee J, Samarasena J. Ethanol Ablation of a Peripheral Nerve Sheath Tumor Presenting as a Small Bowel Obstruction. ACG Case Rep J. 2015;3(1):31-32.
- Abraham AP, Franklyn J, Chandramohan J, Gaikwad P, Muthusami JC. Malignant Peripheral Nerve Sheath Tumour of the Small Bowel Presenting with Intussusception and Perforation: a Double Jeopardy?. Indian J Surg Oncol. 2017; 8: 206-209.
- Zhu LB, Li PF, Xiao WH, Zhang PB, Li JQ, Sun MF. A distal ileum malignant peripheral nerve sheath tumor causing intussusception in a patient in China: a case report. World J Surg Oncol. 2017; 15: 29.
- Lakkasani S, Shaaban HS, Guron G, Fedida A. Primary Malignant Peripheral Nerve Sheath Tumor of Small Bowel and Colon Presenting With Metastases to Bone, Lung, Liver, Spleen, and Brain. Am J Gastroenterol. 2019; 114: S1459.
- Park YS, Lim SJ, Kim WH, Ham EK. Malignant Peripheral Nerve Sheath Tumor in Descending Colon: A Case Report. Korean J pathol. 2002; 36: 179-183.
- Lee YJ, Moon HG, Park ST, Ha WS, Choi SG, Hong SC, et al. Malignant peripheral nerve sheath tumor arising from the colon in a newborn: report of a case and review of the literatures. J Pediatr Surg. 2006; 41: 19-22.
- Marwah S, Gurawalia JP, Sheoran KD, Marwah N, Gupta S, Ranga H. Malignant peripheral nerve sheath tumor of the colon in a patient with von Recklinghausen’s disease: report of a case. Clin J Gastroenterol. 2013; 6: 429-433.
- Cyriac SL, Walia R, Suri V, Bakhshi S. Colonic malignant peripheral nerve sheath tumor in a child. J Pediatr Hematol Oncol. 2014; 36: 661-662.
- Rawal G, Zaheer S, Ahluwalia C, Dhawan I. Malignant peripheral nerve sheath tumor of the transverse colon with peritoneal metastasis: a case report. J Med Case Reports. 2019; 13: 15.
- King AA, DeBaun MR, Riccardi VM, Gutmann DH. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. Am J Med Genet. 2000; 93: 388–392.
- Bates JE, Peterson CR, Dhakal S, Giampoli EJ, Constine. Malignant peripheral nerve sheath tumors (MPNST): a SEER analysis of incidence across the age spectrum and therapeutic interventions in the pediatric population. Pedatr Blood Cancer. 2014; 61: 1955–1960.
- Karatzoglou P, Karagiannidis A, Kountouras J, Christofiridis CV, Karavalaki M, Zavos C, et al. Von Recklinghausen’s disease associated with malignant peripheral nerve sheath thmor presenting with constipation and urinary retention: a case report and review of the literature. Anticancer research. 2008; 28: 3107–3113.
- Benz MR, Czernin J, Dry SM, Tap WD, Allen-Auerbach MS, Elashoff D, et al. Quantitative F18-fluorodeoxyglucose positron emission tomography accurately characterizes peripheral nerve sheath tumors as malignant or benign. Cancer. 2010; 116: 451–458.
- Bees NR, Ng CS, Dicks-Mireaux C, Kiely EM. Gastric malignant schwannoma in a child. Br J Radiol. 1997; 70: 952-955.
- Grobmyer SR, Reith JD, Shahlaee A, Bush CH, Hochwald SN. Malignant Peripheral Nerve Sheath Tumor: molecular pathogenesis and current management considerations. J Surg Oncol. 2008; 97: 340–349.
- Farid M, Demicco EG, Garcia R, Ahn L, Merola PR, Cioffi A, Maki RG. Malignant peripheral nerve sheath tumors. Oncologist. 2014; 19: 193–201.
- Kindblom LG, Ahlden M, Meis-Kindblom JM, Stenman G. Immunohistochemical and molecular analysis of p53, MDM2, proliferating cell nuclear antigen and Ki67 in benign and malignant peripheral nerve sheath tumours. Virchows Arch. 1995; 427: 19–26.
- James AW, Shurell E, Singh A, Dry SM, Eilber FC. Malignant Peripheral Nerve Sheath Tumor. Surg Oncol Clin N Am. 2016; 25: 789–802.
- Anghileri M, Miceli R, Fiore M, Mariani L, Ferrari A, Mussi C, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006; 107: 1065–1074.