
Research Article
Ann Hematol Onco. 2023; 10(1): 1417.
Diagnosing, Imaging and Successfully Treating a Debilitating Case of Bing-Neel Syndrome: A Multidisciplinary Feat
Kerley RN1,2*, O’Donnell N², Lynott F², Mulcahy R² and Hennessy B²
¹Cork University Hospital, Wilton, Cork, Ireland
²University Hospital Waterford, Dunmore Road, Waterford, Ireland
*Corresponding author: Robert N KerleyCork University Hospital, Wilton, Cork, & University Hospital Waterford, Dunmore Road, Waterford, Ireland
Received: January 10, 2023; Accepted: February 17, 2023; Published: February 24, 2023
Abstract
Waldenstroms Macroglobulinaemia (WM) is a rare B-cell lymphoma representing ~2% of all haematological malignancies. While most neurological complications of WM are secondary to the overproduction of Immunoglobulin M (IgM), Bing-Neel Syndrome (BNS) is an extremely rare direct Central Nervous System (CNS) infiltration by malignant Lymphoplasmocytic Lymphoma (LPL) cells. Limited information on BNS exists in the literature with sparse case reports and case series. Here we present a diagnostically challenging BNS case successfully treated with systemic chemoimmunotherapy and ibrutinib, with remarkable clinical response.
Introduction
A 63-year-old lady presented to the Emergency Department (ED) with a one-day history of binocular diplopia and evidence of abducens nerve palsy on examination. Medical history included dyslipidaemia, non-alcoholic fatty liver disease (NASH) and colonic poly removal. Visual symptoms occurred gradually over weeks without diurnal variation. Fundal examination was unremarkable. Admission bloods, chest x-ray and CT brain did not reveal a cause for the patient’s neurological symptoms. Full blood count showed haemoglobin (Hb) of 10.6 g/dL but was otherwise unremarkable. Her vasculitic, viral, and lyme serology screen were negative. Incidentally, her serum protein electrophoresis showed an IgM paraproteinemia, with a paraprotein level of 4.2 g/L. Renal function, serum calcium, beta-2-micorglobulin and plasma viscosity levels were within normal ranges. An MRI Brain, orbits and cervical cord revealed significant cervical spondylosis with multi-level nerve root impingement. Lumbar Puncture (LP) showed raised Cerebrospinal Fluid (CSF) protein (0.97 g/dL) and leukocyte count (31 x 109/L, 90% lymphocytes, 10% polymorphs). She was discharged with an incidental diagnosis of Monoclonal Gammopathy of Undetermined Significance (MGUS) with close follow from neurology and general medicine.
Progression and Diagnosis
Two weeks later the patient was re-admitted with ataxia, gait disturbance and loss of power bilaterally in her lower limbs. On examination, power was 3/5 in both lower limbs and 5/5 in her upper limbs. Tone was normal, sensation and reflexes were intact without evidence of coordination deficits and an extensor plantar reflex. A repeat MRI Brain and whole spine with contrast was negative for leptomeningeal or parenchymal enhancement. Without a diagnosis and progressive neurological deficits, a broader differential was conducted to include nerve conduction studies, a repeat autoimmune, vasculitic and viral screen, CT thorax, abdomen & pelvis, and serum ACE all of which were non-diagnostic. Finally, a repeat LPwith CSF immunophenotyping and second MRI whole spine with contrast were ordered to exclude infiltrative disease (Figure 1). This MRI result was discussed at three major neuroradiology sites in our country to form a consensus on the presence of infiltrative disease. Subtle posterior meningeal enhancement involving the distal cord at T10 and T11 vertebral bodies was observed. In the context of macroglobulinaemia, a bone marrow biopsy was performed which showed a normocellular specimen with no evidence of lymphomatous infiltration. While awaiting her CSF sample to return from flow cytometry she deteriorated with ascending paralysis compromising respiratory drive and requiring transfer to the Intensive Care Unit (ICU). The following morning, CSF immunphenotyping revealed a clonal population of kappa light chains restricted B cells, which were CD5 and CD10 negative, consistent with CNS involvement by lymphoma, in keeping with a diagnosis of BNS.
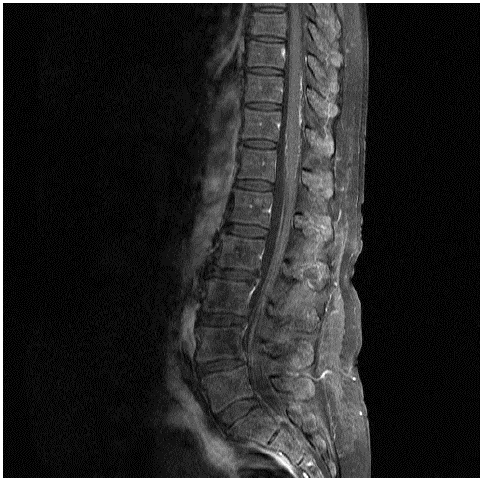
Figure 1: MRI whole spine outlining subtle posterior meningeal enhancement at T10 and T11.
Treatment
Prior to diagnosis, IV Methylprednisolone 1 g was given for three days with a partial subjective response to limb paralysis but no response as regards her abducens nerve palsy. Following CSF analysis, a combination of chemo- and immunotherCitationapy with Intrathecal (IT) Methotrexate (MTX), subcutaneous Rituximab and pulsed oral dexamethasone were urgently commenced. Twenty-four hours post IT-MTX, Ibrutinib 420 mg PO OD was introduced. Due to the patient’s neurological deficits and difficulty performing LPs, as well as reluctance to interrupt ibrutinib for LPs, a decision was made to switch from IT-MTX to high dose IV MTX (3.5g/m²) for second and third cycles. In total the patient received the following induction therapy: one IT MTX, two cycles of high dose IV MTX, weekly rituximab for four weeks, four pulses of oral dexamethasone (40mg fortnightly) and oral ibrutinib. This treatment was well-tolerated. A repeat CSF sample was sent for flow cytometry post the above treatmentwith no clonal population of B cells detected. In addition, the patient’s neurological symptoms responded over weeks from 1/5 power to 4/5 power in her lower limbs. As per the 2014 task force guidelines on BNS these CSF findings in conjunction with the resolution of symptoms suggests a clinical response in keeping with complete remission [1]. The patient received two further doses of subcutaneous rituximab monthly. She currently remains on oral ibrutinib, with a plan to continue this for at least two years.
Discussion
The clinical presentation of BNS as shown in this case is diverse and non-specific. A critical point that could explain the under recognition of BNS is its frequent occurrence independent of any systemic progression of WM, with up to one-third of BNS cases coinciding with diagnosis of WM [2,3]. Radiological assessment was critical to the diagnosis in this case requiring input from experienced neuroradiologists. In a case series of 24 patients the most common MRI finding was leptomeningeal infiltration either intracranial or spinal with a prevalence reaching 70.8% [4]. Dural and parenchymal involvements were present in 37.5% and 41.7% of patients, respectively. Autopsy results have observed more extensive meningeal and perivascular infiltration by malignant cells than that revealed by MRI [5]. the gold standard for diagnosis is a histological biopsy of the affected area or CSF analysis by flow cytometry demonstrating malignant cells as previously outlined [1].
There is no established treatment regimen for BNS to date. Patients typically receive a combination of systemic and IT chemotherapy, immunotherapy, and novel agents, such as ibrutintib, an oral Bruton kinase inhibitor. The goal of treatment should be to (1) reverse the patient’s clinical symptoms and (2) induce prolonged progression-free survival [6]. Remission has been reported with both IT therapies and/or systemic chemotherapies [1,2,7,8]. Use of high-dose MTX is extrapolated from data in primary CNS lymphoma while direct evidence supports the use of Rituximab in BNS [3,9]. Ibrutinib, has been increasingly employed in BNS treatment, as well as other primary CNS lymphomas [10-12]. Data suggests ibrutinib maintenance also has a role in prolonging progression-free survival rationalising its use in our patient’s case [13]. In one review of 34 patients with BNS, the estimated overall survival rate at 3 years was 59% [14]. Age >65 years, thrombocytopenia and previous treatment for WM were all associated with worse prognosis [14]. Our patient has none of these characteristics and remains in complete remission 6 months post diagnosis.
References
- Minnema MC, Kimby E, D’Sa S, Fornecker L-M, Poulain S, Snijders TJ, et al. Guideline for the diagnosis, treatment and response criteria for Bing-Neel syndrome. Haematologica. 2017; 102: 43-51.
- Varettoni M, Defrancesco I, Diamanti L, Marchioni E, Farina LM, Pichiecchio A. Bing-Neel Syndrome: Illustrative Cases and Comprehensive Review of the Literature. Mediterranean journal of hematology and infectious diseases. 2017; 9: e2017061-e.
- Simon L, Fitsiori A, Lemal R, Dupuis J, Carpentier B, Boudin L, et al. Bing-Neel syndrome, a rare complication of Waldenström macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO). Haematologica. 2015; 100: 1587-94.
- Fitsiori A, Fornecker LM, Simon L, Karentzos A, Galanaud D, Outteryck O, et al. Imaging spectrum of Bing-Neel syndrome: how can a radiologist recognise this rare neurological complication of Waldenström’s macroglobulinemia? Eur Radiol. 2019; 29: 102-14.
- Matsuda S, Sekiguchi N, Ito K, Takaoka K, Furuki M, Hirano K, et al. An Autopsy Case of Bing-Neel Syndrome: Discrepancy between the Radiological and Pathological Findings. Intern Med. 2019; 58: 1947-51.
- Castillo JJ, Treon SP. How we manage Bing–Neel syndrome. British Journal of Haematology. 2019; 187: 277-85.
- Nagaharu K, Miyazami K, Imai H, Tamura A, Umino A, Fujieda A, et al. Successful treatment of Bing-Neel syndrome using combination therapy with fludarabine and rituximab. Rinsho Ketsueki. 2014; 55: 2423-8.
- Gavriatopoulou M, Ntanasis-Stathopoulos I, Moulopoulos LA, Manaios A, Fotiou D, Eleutherakis-Papaiakovou E, et al. Treatment of Bing-Neel syndrome with first line sequential chemoimmunotherapy: A case report. Medicine (Baltimore). 2019; 98: e17794.
- Poulain S, Boyle EM, Roumier C, Demarquette H, Wemeau M, Geffroy S, et al. MYD88 L265P mutation contributes to the diagnosis of Bing Neel syndrome. Br J Haematol. 2014; 167: 506-13.
- Bernard S, Goldwirt L, Amorim S, Brice P, Brière J, de Kerviler E, et al. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood. 2015; 126: 1695-8.
- Chamoun K, Choquet S, Boyle E, Houillier C, Larrieu-Ciron D, Al Jijakli A, et al. Ibrutinib monotherapy in relapsed/refractory CNS lymphoma: A retrospective case series. Neurology. 2017; 88: 101-2.
- Cabannes-Hamy A, Lemal R, Goldwirt L, Poulain S, Amorim S, Pérignon R, et al. Efficacy of ibrutinib in the treatment of Bing–Neel syndrome. American Journal of Hematology. 2016; 91: E17-E9.
- O’Neil DS, Francescone MA, Khan K, Alobeid B, Bachir A, O’Connor OA, et al. A Case of Bing-Neel Syndrome Successfully Treated with Ibrutinib. Case reports in hematology. 2018; 2018: 8573105.
- Castillo JJ, D’Sa S, Lunn MP, Minnema MC, Tedeschi A, Lansigan F, et al. Central nervous system involvement by Waldenström macroglobulinaemia (Bing-Neel syndrome): a multi-institutional retrospective study. Br J Haematol. 2016; 172: 709-15.