
Research Article
Ann Hematol Onco. 2023; 10(2): 1418.
Management of Cancer Patients with Opioid-Induced Constipation (OIC): The Experience of Three Italian Centres
Guardamagna V¹*, Salé EO¹, Paolo MDP², Nardulli P³, Giotta F³ and Gini G²
¹Istituto Europeo di Oncologia, Milan, Italy
²AOU Ospedali Riuniti, Ancona, Italy
³IRCCS Istituto Tumori Giovanni Paolo II, Bari, Italy
*Corresponding author: Guardamagna VIstituto Europeo di Oncologia, Milan, Italy
Received: January 10, 2023; Accepted: February 24, 2023; Published: March 03, 2023
Abstract
Objectives: To present the Training on the field about OIC undertaken by three Italian oncological centres involving professionals of different sub-specialties.
Methods: Four on-site interactive meetings of multidisciplinary hospital teams; interactive presentation and discussion, questionnaires administration at the baseline and at the end of the field programme over two years (2019-2021). Outcomes evaluated by estimated increase of OIC diagnosis and OIC treatments.
Results: Shared principles about OIC prevention and management in all the steps (from laxatives to PAMORAs), and use of PROMs.
Participants were asked about their initial awareness at the inception of the Training on the field programme and their final experiences at the end.
Discussion: The experience of this Training on the field underlined the cardinal role of a constant teamwork in the Onco-ematology and Palliative Care Departments and the territory with a shared and tested algorithm. The proposed platform of activities is a call to action to mitigate the burden of pain and OIC if specialists act in concert during all the steps of the trajectory of cancer patients requiring opioid treatment. Multidisciplinary teamwork may help redefine and optimize doses and time schedules tailored to each patient and can contribute to designing controlled studies of OIC
Introduction
Opioid-Induced Constipation (OIC) increases the suffering of cancer patients and can interfere with opioid therapies for cancer pain [1-4]. OIC is engendered by the agonism of opioid receptors in the gastrointestinal tract leading to reduced intestinal secretion and motility [5]. Although variable, the OIC prevalence in the populations of cancer patients is high (60-90%) [6,7]. OIC increases with age (e.g., in older patients, it can be five times more than in younger subjects) and in those undergoing palliative care was reported as 51-55% [7,8]. OIC does not depend on the opioid type and dose; thus, patients do not develop tolerance [9]. Although common and burdensome, OIC is overlooked and consequently undertreated [10]. The need for extended care for OIC is arising, especially with the increased use of opioids for pain therapy. In Italy, in 2014, the number of Defined Daily Doses (DDD) was 6.9, while in 2020; it was 7.6, with an increase of 1.7% [11].
The lack of a standardized definition of OIC may hinder the early detection of the disorder, even among specialists. The Rome IV criteria were set for Functional Gastrointestinal Disorders (FGIDs) and, in 2016, included OIC among the bowel disorders of chronic constipation as “a constipation triggered or worsened by opioids analgesics” [12]. Given that the clinical presentation of OIC and other FGIDs can be similar, a differential diagnosis is required [7,13]. Common misalignments in the perception and communication between healthcare providers and patients further hinder the improvement of OIC management and sustainment of optimal pain therapy [14-16].
Although some controlled trials have been recently published, the general level of evidence about OIC is still moderate or low. This limited evidence about OIC may explain the high number of expert consensus publications about best practices [7] and entails the need for studies with high-quality designs and structured analyses of local experiences provided by oncological specialists. Also, educational programmes devoted to OIC aimed at sharing experiences can increase awareness and optimize management [10]. The present study described the experience of a field training programme about OIC undertaken by three Italian centres that involved professionals of different sub-specialties in charge of oncological patients.
Materials and Methods
The present study was conducted according to the World Medical Association’s Declaration of Helsinki (1964, version 2013) [17] and Good Clinical Practice. The medical institutional board of IEO, AOU Ospedale Riuniti, and IRCCS Istituto Tumori Giovanni Paolo II approved this study.
Programme Structure
The Training on the field was performed on-site in four interactive meetings and targeted multidisciplinary teams of professionals working in oncological or palliative care centres and general practitioners involved in the pathways of diagnosis and care of OIC.
Interactive presentations performed by the experts of the three reference centres set the stage to open discussions about employing the most acknowledged guidelines, recommendations, and best practices of the consensus of advisory boards selected by the dedicated literature review. The follow-up was performed by simple questionnaires about the awareness of OIC, concepts or criteria of OIC management, knowledge and application of selected assessment, diagnostic, and monitoring tools for qualitative data to measure the outcomes.
The outcomes of the Training on the field were evaluated by the estimated increased OIC diagnosis, nutritional and lifestyle suggestions, and use of OIC treatments during the following months after the programme.
A literature search was conducted to select the most recent publications about OIC management focused on cancer patients and comparing the practical suggestions. The literature search was based on the database of the National Institutes of Medline (PubMed) and a manual search of the most recently published guidelines, recommendations, consensus, and best practices about OIC in cancer patients over the previous ten years. The search terms series included opioid-induced constipation, cancer, and OIC guidelines.
Results
During the 2019-2021 periods, three Italian oncological centres were involved as expert centres in a field training programme for the practical management of OIC. The participants to the programme were:
• Oncologists: 15
• Haematologists: 3
• Pain therapy specialists: 4
• Radiotherapist: 1
• General practitioners (GPs): 4
• Clinical pharmacists: 7
• Nurses: 21
All participants acknowledged OIC as a central problem given the discomfort for patients with cancer, the delayed recovery, and discharge from the hospital. At the baseline, most of the participants felt inadequate for OIC management.
Prophylaxis and Treatments
Participants acknowledged lifestyle changes as an essential suggestion for the patient to prevent or lessen OIC during the opioid treatment. The shared suggestions furthered prolonging prophylaxis with laxatives during opioid therapy, especially for patients at high risk of developing OIC. For the OIC prophylaxis, most participants indicated a preference for osmotic laxatives and, as a first-line treatment for the management of OIC, a combination of stimulant laxatives. The therapeutic features of laxatives for the prevention and management of OIC were outlined and shared among the participants:
• Mass laxatives: increase residual in the colon, reabsorb water, stimulate the propulsion;
• Emollients and lubricants: enhance the link with water in the bowel lumen to allow a more interaction of the solid faeces with water, thus easing the passage of the faeces;
• Osmotic laxatives: draw water in the bowel according to the osmotic gradient;
• Stimulant laxatives: act on the colon, stimulate the intestinal motility or decrease the absorption of fluids in the colon, favour the secretions.
At the end of the programme, participants shared other principles for the management of OIC. In the case of inefficacy, the laxative could be substituted or associated with another one of different categories. However, the use of laxatives is often insufficient for OIC management and can cause gastrointestinal side effects. In patients who are refractory to laxatives, the OIC treatment guidelines include the prescription of Peripheral Mu-Opioid Receptor Antagonists (PAMORAs). Therefore, in the case of diagnosis of OIC, the participants in the field training considered: a) a combination of two laxatives and b) a PAMORA plus a laxative. If the PAMORA treatment fails, the most common rescue medications were evacuative enemas or glycerine, followed by Senna or Bisacodyl, or “others”.
PROMs
As symptom is defined a “subjective perception expressed with a high level of subjectivity”, the patient is the most suitable subject to evaluate the symptoms. The Edmonton Symptom Assessment System (ESAS) is a simple and multidimensional tool that can be used quickly and assure the patients' compliance even in general compromised conditions. The ESAS scale was modified by including OIC and inserted in the clinical charts of the patients with diagnosed OIC. Also, a questionnaire for patients was added to the clinical chart with adjustments according to the local settings. Once defined and started the therapy for constipation, the Bristol Stool Form Scale (BSFS) can be a helpful diagnostic tool for patient monitoring (Figure 1). The BSFS enables the categorization form and compactness of human faeces. The form and compactness of the faeces and their permanence time in the colon are statistically correlated.
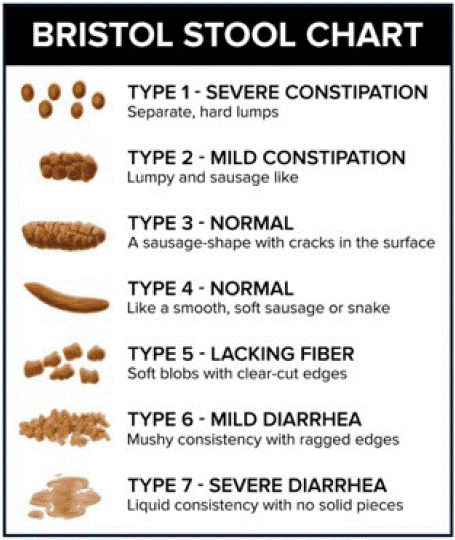
Figure 1: Proposed PROM assessment picture of OIC according to the BSFS.
Outcomes
At the baseline of the field training, the prescription level of PAMORAs was high in the subgroup of pain therapy specialists and increased after the training. We observed a moderate increase in PAMORAs prescription even among GPs.
The field training showed a slow but remarkable advancement in standardizing the diagnosis of OIC, assessing and monitoring bowel function, and prescribing the recommended treatments. The emerged statements were:
• The OIC therapy should be individualized;
• The OIC therapy should consider the clinical conditions of the patients, comorbidities, concomitant pharmacological treatments, objective examination, and possible clinical/radiological examinations;
• The use of conventional laxatives (osmotic or stimulants) is advisable as a first-line for prevention and treatment of OIC;
• The choice of the laxative should ground on a careful evaluation of the patient and the tolerance of the treatment;
• Periodic assessments of the patient are needed;
• If two laxatives fail, a PAMORA should be considered as a therapeutic solution.
At the end of the field training, the participants further marked the importance of the early OIC diagnosis as predictive of the outcomes and should refer to the Rome IV criteria. Participants deemed essential PROMs based on validated, easy-to-use, quick tools, such as BSFS and modified ESAS.
After the training, a regular system to evaluate bowel function was set up in the involved centres for all the patients undergoing opioid treatments and employing standardized assessment scales.
During the field training, relevant differences emerged between the OIC management and the monitoring of in-hospital patients, outpatients, or day-hospital patients based on the experience of doctors and nurses. The detection and monitoring were easier for in-hospital, outpatient, or day-hospital patients who often use non-standardized treatments.
Algorithm
We synthesized the pathways of management of OIC for routine daily practice. The algorithm was based on the experience of the three reference centers and a synthesis of the evidence, guidelines, and recommendations about the detection and diagnostic replicable tools and treatment of OIC. This synthesis was used for the proposed OIC protocol expressed by the algorithm in (Figure 2).
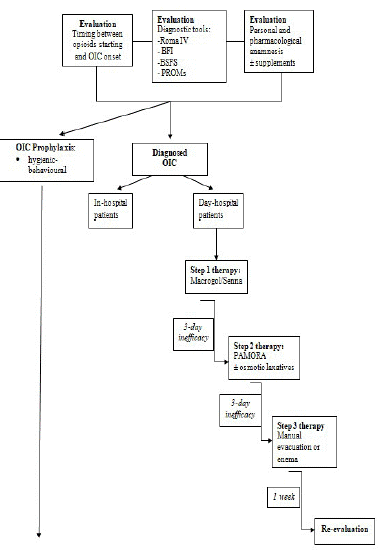
Figure 2: Proposed algorithm for the practical management of OIC.
Activities Platform
Following the experience of the field training, we also proposed a structured platform of activities that involves the multidisciplinary oncological teams modulated in three steps (Figure 3):
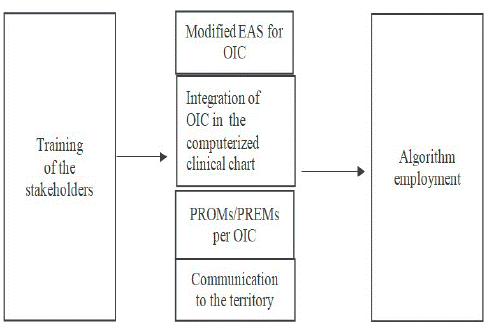
Figure 3: Visualization of the proposed structured platform of activities for OIC management.
• Step 1: meetings and constant training of all the stakeholders regularly(e.g., monthly);
• Step 2: employment of the series of different tools (modified EAS, PROMs, etc.), filling the computerized clinical chart with OIC data, regular communication with the territory (GPs);
• Step 3: steady application and adaptation of the algorithm.
Discussion
The results of this study have highlighted the importance of field training for multidisciplinary teams to increase awareness about OIC and real-world best practices to improve management from the anamnesis, prevention, and diagnosis to treatments of the disorders expressed in an actionable algorithm. Applying our structured platform of activities entails the early involvement of the patients and caregivers and a specific communication plan with the territory, especially with GPs.
OIC and constipation exacerbated by opioids require a differential diagnosis. Medications associated with opioids or systemic pathologies can be the ideal substrate for developing constipation. Opioid treatments might not cause constipation but exacerbate it. Antidepressants, anticonvulsants, antihistaminic drugs, diuretics, calcium antagonists, antiemetics are all able to induce constipation. In these cases, adding an opioid can have a synergic effect in bringing to light possible borderline constipation or exacerbating the pre-existent one. Moreover, possible pre-existing or concomitant clinical conditions as causes or risk factors of constipation should be investigated. There could be mechanical conditions related to the gastroenteric anatomy and function, neurologic diseases, and metabolic disturbances (e.g., electrolyte abnormalities) [8,18,19]. These conditions make the OIC presentation highly variable.Constipation requires an examination from the first visit, the assessment of risk factors, and possible previous use of non-specific self-prescribed remedies, which may entail severe consequences [20].
The OIC management should balance prevention and self-care and prescribe oral and rectal laxatives. Moreover, it is advisable to distinguish between the patients undergoing oncological therapies and those with palliative care, given that lifestyle factors lose importance progressively over time. When possible, avoiding drug-drug interaction is also recommended [7].
The OIC diagnosis cannot be based on empiric criteria. Instead, the Rome IV criteria have systematized the OIC definition as the onset or worsening of symptoms of constipation at the beginning or switch or increase of opioid therapy, associated with two or more of the following conditions:
• strengths during more than ¼ (25%) of defecations;
• lumpy or hard or stools (BSFS 1-2) in more than ¼ (25%) of defecations;
• sensation of incomplete evacuation of more than ¼ (25%) of defecations;
• sensation of anorectal obstruction/blockage of more than ¼ (25%) of defecations;
• manual manoeuvres to facilitate more than ¼ (24%) of defecations;
• Fewer than three intestinal spontaneous movements (SBM) per week.
According to the Rome IV criteria, the OIC diagnosis can be established when two or more out of the aforementioned six statements are fulfilled and when there is no diarrhoea in the absence of laxatives. However, the hurdles of OIC diagnosis and management are also due to the different local hospitals and territorial organizations. Therefore, the various organizational patterns demand easy-to-use diagnostic and monitoring tools flexible for each patient with OIC.
The use of assessment scales and validated PROMs proved to be advantageous for OIC diagnosis and monitoring. Specific constipation assessment scales, such as the Bristol Stoll Form Scale (BSFS), which is composed of images, can provide a clinical benefit to patients with advanced cancer and OIC [7]. Among the currently validated assessment tools, the Bowel Function Index (BFI) and the Stool Symptom Screener (SSS) showed the best utility and are the most applied [7]. An Italian expert panel recommended using the BFI alone for screening and BSFS to confirm the diagnosis and laxatives for patients with BSFS stool types 1-3 to obtain types 4 and 5 stools [19]. However, another expert panel has suggested integrating the BFI evaluations (ease of defection, a sensation of incomplete bowel evacuation, personal assessment of constipation) with registering the frequency of bowel movements and rectal tenesmus [20].
As a consensus about the numerous rating scales for assessing OIC has not yet been achieved, it is unlikely that a single scale can suit all the different types of the disorder and settings [21]. The combination of three measures (BSFS for an objective assessment, BFI to integrate PROMs, and the patients’ burden of OIC) could be the best solution. The Gastrointestinal Symptom Rating Scale is a validated questionnaire, possibly associated with patients’ diaries for information about bowel movements, pain, stool consistency, and laxatives [21].Based on three questions, the numerical scale BFI is one of the most common and manageable to evaluate the treatment. The Patient Assessment of Constipation Quality of Life questionnaire (PAC-QOL) is composed of 28 questions; it helps assess the constipation quality of life and is subdivided into scores related to physical, psychosocial, worries/concerns, satisfaction subscales, and an overall score. The Memorial Symptom Assessment Scale-short form (MSAS-SF) has 32 PROM items to evaluate physical and psychological symptoms [22]. A prospective non-randomized interventional study involved 100 cancer patients with OIC diagnosed according to the Rome IV criteria to evaluate the application of a four-step algorithm for OIC management [22]. The patients were assessed by BFI, PAC-QOL, and MSAS-SF at the time of enrollment and on days 7,14,21, and 28. The results of this feeder study showed the importance of regular assessments of cancer patients with OIC by PROMs, with improved BFI associated with PAC-QOL score. The algorithm's utility in managing OIC showcased clinical improvements to increase the patients' adherence to treatments [22]. The self-rated scale ESAS, which measures the severity of nine common symptoms by clinicians, was validated in 202 outpatients with advanced cancer. It added Constipation and Sleep (CS) disturbance (ESAS-r-CS) and a revised version of its Numerical Rating scale (NRS) (ESAS-r-CS NRS) [23]. ESAS-r-CS and ESAS-r-CS NRS scales demonstrated reliability for measuring symptoms in this population of outpatients, especially with the 24-hour time frame of the ESAS-r-CS as it provided a clear track of fluctuating symptoms [23].
Regarding treatments,opioids and osmotic or stimulant laxatives should be co-prescribed to all patients, excluding those with prior diarrhoea [7]. The observational study of Neefjes et al. (2019) on 327 patients with advanced-stage and under opioid treatment has highlighted the contribution of timely intensification of the laxatives for OIC prevention; in particular, when an increase of opioid doses is required [24]. However, when OIC persists, PAMORAs administration is currently the best option [7]. The efficacy and safety profiles of PAMORAs have been bolstered by a series of randomized controlled clinical trials and systematic reviews [25-27]. In Italy, PAMORAs have been approved for OIC since April 2020 in patients classified according to Rome IV criteria with chronic and continuous therapy with opioids and OIC resistant to two laxatives (one of them osmotic) treatment after three days. Therefore, the use of PAMORAs has been extended beyond the patients at the terminal stage. The Italian Association of Medical Oncology (AIOM)'s guidelines underscored the efficacy of PAMORAs in reducing constipation without influencing the antalgic central effect and with a favourable toxicity profile and optimal tolerance [28,29]. PAMORAs allow avoiding the dose reduction or withdrawal of the opioids that can involve one-third of the patients [21], thus allowing the proceeding in having benefits for pain. Also, the meta-analysis of Nee et al. (2018) that included 23 randomized clinical trials about the evaluation of the approved PAMORAs showed their efficacy and safety compared with placebo (RR 0.69, 95% IC 0.62-0.77) [30]. The PROMs, such as PAC-QOL, can help assess PAMORAs treatments for OIC in cancer patients in real-world settings. In an observational study, PAC-QOL and Patients Assessment of Constipation Symptoms (PAC-SYM) questionnaires significantly improved after 15 days of treatment with naloxegol in 126 cancer patients with a good safety profile followed-up for three months(p<0.001) [31].The PAMORAs currently available are three: methylnaltrexone, naldemedine, and naloxegol. Naldemedine and naloxegol can be administered per os, whereas methylnaltrexone subcutaneously. Moreover, the naloxegol tablets can be crushed in water and administered by mouth or nasogastric tube, and these modalitiescan benefits especially patients with dysphagia [32]. In a non-interventional French multicenter study that enrolled 124 patients with different types and stages of cancer, the treatment with naloxegol was tied to a clinically relevant decrease of PAC-QOL score ≥ 5 at week 4 [33].
All the stakeholders, including clinicians, oncology nurses, clinical pharmacists, GPs, and patients' family has equal importance to OIC management according to their specific roles and function. Inside the multidisciplinary team, the clinical pharmacists play an essential role in counselling the prescriptive appropriateness of the pharmacological therapies according to the evolution of the patient's conditions. The partnership of clinical pharmacists with the oncology teams is based on several functions: a) sharing the patients’ needs for pharmacological interventions, b) optimizing the pharmacological therapies and providing suggestions about their implementations, c) collaborating in the monitoring the adherence to therapies; d) recording the pre-existing therapies and the pharmacological interventions in the computerized clinical chart; e) promoting the treatment continuity with the territory. Drug-drug and drug-food interactions are other fields pertinent to the clinical pharmacist role. Oncology nurses play a crucial role in the comprehensive assessment and monitoring of OIC in the hospital, in patient-clinician communication, and the communication with caregivers and family members [34-36]. Recently, the involvement of GPs in OIC management has been underscored by an expert’s board that focused on increasing awareness through an early multidisciplinary approach, the optimal management therapies for in-hospital and outpatients [20].
The algorithm illustrated in (Figure 3) intends to propose a simple and comprehensive framework for the detection and management of OIC. The algorithm was based on the literature and the experience of the experts of the three centers, and shared with the participants of the field training programme. According to the practical ESMO recommendations about OIC, all cancer patients should be evaluated for constipation; the assessment should include a question to identify its causes, and the use of PROMs is recommended. In the case of OIC diagnosis, physical examinations are also recommended [7]. The different modalities in monitoring and management of OIC between in-hospital and out or day-hospital patients lead to differentiating the use of the tools (PROMs).
The authors of the present study suggested inserting the OIC parameters in the computerized clinical chart as a crucial practice to get used to all the involved stakeholders. Including OIC in the clinical chart can ease its integration into the routine clinical practice of pain management with opioids. From a perspective of a shared plan of care for cancer patients with OIC, the employment of the digitized chart is essential in tracking the interventions of the various healthcare providers.
The platform of activities considers the complex and manifold clinical presentations of OIC and the impact on the quality of life of cancer patients and their caregivers. Constant training about the application of all the available tools is thus mandatory.
Therefore, adequate and steady education and communication with the patients about the use of drugs (opioids) may ease the avoidance of the suspension of the opioid treatment and the new onset of symptoms. The tools' dashboard of the proposed platform of activities can be integrated and adapted according to the local organizational healthcare setting.
The limitations of the present study are related to the lack of quantitative analysis; however, it means to increase awareness about OIC and propose a more systematic approach to the OIC problem in Italian settings.
Further investigations in the field can include stratification systems according to the risk of developing OIC and different levels of application of guidelines. Moreover, future studies can also deepen the importance of diet and lifestyle, laxatives and over-the-counter medications, and the role of patient education [37].
Conclusions
The experience of this field training program underlined the cardinal role of routine teamwork inside the centres specialized in oncology or haematology, palliative care, and with the territory applying a shared and tested algorithm. The proposed activities platform is a call to action. In fact, the burden of pain and OIC may be mitigated if only the specialists act in concert during all the steps of the trajectory of cancer patients requiring opioid treatment. Multidisciplinary teamwork may help redefine and optimize doses and time schedules tailored to each patient and can contribute to designing controlled studies of OIC.
References
- Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, et al. The prevalence, severity, and impact of opioid-induce bowel dysfunction: results of a US and European patient survey (PROBE 1). Pain Med. 2009; 10: 35-42.
- Rumman A, Gallinger ZR, Liu LWC. Opioid induced constipation in cancer patients: pathophysiology, diagnosis and treatment. Expert Review of Quality of Life in Cancer. 2016; 1: 25-35.
- Corli O, Santucci C, Corsi N, Radrezza S, Galli F, et al. The burden of opioid adverse events and the influence on cancer patients’ symptomatology. J Pain Symptom Manage. 2019; 57: 899-908.
- Varrassi G, Banerji V, Gianni W, Marinangeli F, Pinto C, et al. Impact and consequences of opioid-induced constipation: a survey of patients. Pain Ther. 2021; 10: 1139-1153.
- Aziz I, Whitehead WE, Palsson OS, Tornblom H, Simren M, et al. An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation. Expert Rev Gastroenterol Hepatol. 2020; 14: 39-46.
- Bruner HC, Atayee RS, Edmonds KP, Buckholz GT. Clinical utility of naloxegol in the treatment of opioid-induced constipation. J Pain Res. 2015; 8: 289-94.
- Larkin PJ, Cherny NI, La Carpia D, Guglielmo M, Ostgathe C, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018; 29: iv111-iv125.
- Liao SS, Slatkin NE, Stambler N. The influence of age on central effects of methylnalthrexone in patients with opioid-induced constipation. Drugs Aging. 2021; 38: 503-511.
- Saha S. Nathani P, Gupta A. Preventing opioid-induced constipation. A teachable moment. JAMA Intern Med. 2020; 180: 1371-1372.
- Alvaro D, Coluzzi F, Gianni W, Lugoboni F, Marinangeli F, et al.Opioid-induced constipation in real-world practice: a physician survey, 1 year later. Pain Ther. 2022; 11: 477-491.
- Osservatorio Nazionale sull’Impiego dei Medicinali (OsMed). L’uso dei farmaci in Italia. Rapporto nazionale. Anno 2020.
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology. 2016; 150: 1262-1270.
- AL Mouaalamy N. Opioid-induced constipation in advanced cancer patients. Cureus. 2021; 13: e14386.
- Keller MS, Jusufagic A, Spiegel BMR. Patient and provider differences in the treatment of opioid-induced constipation: a qualitative study. BMC Gastroenterol. 2019; 19: 182.
- LoCasale RJ, Datto C, Wilson H et al. The burden of opioid-induced constipation: discordance between patient and health care provider reports. J Manage Care Spec Pharm. 2016; 22: 236-45.
- Coluzzi F, Alvaro D, Caraceni AT, Gianni W, Marinangeli F, et al. Common clinical practice for opioid-induced constipation: a physician survey. J Pain Res. 2021; 14: 2255-2264.
- World Medical Association. World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013; 310: 2191-2194.
- Wald A. Constipation; advances in diagnosis and treatment. JAMA. 2016; 315: 185-91.
- De Giorgio R, Zucco FM, Chiaroni G, Mercadante S, Corazziari ES, et al. Management of opioid-induced constipation and bowel dysfunction: expert opinion of an Italian multidisciplinary panel. Adv Ther. 2021; 38: 3589-3621.
- Rossi M, Casale G, Badiali D, Aielli F, Spiriti MAA, Arcioni R, et al. Opioid-induced bowel dysfunction: suggestions from a multidisciplinary expert board. Support Care Cancer. 2019; 27: 4083-4090.
- Drewes AM, Munkholm P, Simren M, Breivik H, Kongsgaard UE, et al. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction-Recommendations of the Nordic Working Group. Scand J Pain. 2016; 11: 111-122.
- Davies AN, Leach C, Butler C, Patel SD, Shorthose K, et al. Opioid-induced constipation: a stepwise treatment algorithm feasibility study. BMJ Support Palliat Care. 2021; bmjspcare-2020-002754.
- Hannon B, Dyck M, Pope A, Swami N, Banerjee S, et al. Modified Edmonton Symprom assessment including constipation and sleep: validation in outpatients with cancer. J Pain Manage. 2015; 49: 945-52.
- Neefjes ECW, van der Wijngaart H, van der Vorst MJDL, Oever DT, van der HJ, et al. Optimal treatment of opioid induced constipation in daily clinical practice – an observational study. BMC Palliative Care. 2019; 18: 31.
- Schwenk ES, Grant AE, Torjman MC, McNulty SE, Baratta JL, et al. The efficacy of peripheral opioid antagonists in opioid-induced constipation and postoperative ileus. Reg Anesth Pain Med. 2017; 42: 767-777.
- Nishie K, Yamamoto S, Yamaga T, Horigome N, Hanaoka M. Peripherically acting m-opioid antagonist for the treatment of opioid-induced constipation: systematic review and meta-analysis. J Gastroenterol Hepatol. 2019; 34: 818-829.
- Esmadi M, Ahmad S, Hewlett A. Efficacy of naldemedine for the treatment of opioid-induced constipation: a meta-analysis. J Gastrointestin Liver Dis. 2019; 28: 41-46.
- Associazione Italiana Oncologia Medica (AIOM). Terapia del dolore in oncologia. 2021.
- Vijayvargiva P, Camilleri M, Vijayvargiya P, Erwin P, Murad MH, et al. Systematic review with meta-analysis: efficacy and safety of treatments for opioid-induced constipation. Aliment Pharmacol Ther. 2020; 52: 37-53.
- Nee J, Zakari M, Sugraman MA, Whelan J, Hirsch W, et al. Efficacy of treatments for opioid-induced constipation: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018; 16: 1569-1584.
- Cubo Dols MC, Zambrano CB, Gutierrez LC, Sett RC, Lopez-Bara jas MIB, et al. Efficacy of naloxegol on symptoms and quality of life related to opioid-induced constipation in patients with cancer: a 3-month follow-up analysis. BMJ Supportive & Palliative Care. 2021; 11: 25-31.
- Pergolizzi JV Jr, Christo PJ, LeQuang JA, Magnusson P. The use of peripheral-opioid receptor antagonists (PAMORA) in the management of opioid-induced constipation: an update on their efficacy and safety. Drug Des Devel Ther. 2020; 14: 1009-1025.
- Lemaire A, Pointreau Y, Narciso B, Piloquet FX, Braniste V, et al. Effectiveness of naloxegol in patients with cancer pain suffering from opioid-induced constipation. Support Care Cancer. 2021; 29: 7577-7586.
- Palos GR. Opioids and cancer survivors: issues in side-effect management. Oncol Nurs Forum. 2008; 35: 13-9.
- Kurtin S, Fuoto A. Pain management in the cancer survivor. Semin Oncol Nurs. 2019; 35: 284-290.
- Ginex PK, Arnal C, Ellis D, et al. Translating evidence to practice. Clin J Oncol Nurs. 2021; 25: 721-724. https://pubmed-ncbi-nlm-nih-gov.bibliosan.idm.oclc.org/34800103/.
- Stern T, Davis AM. Evaluation and treatment of patients with constipation. JAMA. 2016; 315: 192-3.