
Case Series
Ann Hematol Onco. 2023; 10(2): 1421.
MGMT Methylated High Grade Glioma with Distant Recurrence and Stable Original Tumor Site: Case Series
Jonathan Lee1; Rimas V Lukas2,3; Ignacio Jusue-Torres1; Anand V Germanwala1,4,5; Ewa Borys6; Abhishek A Solanki7; Atul K Mallik8; Kevin Barton9; Jigisha P Thakkar 1,10
1Department of Neurological Surgery, Loyola University Medical Center/Stritch School of Medicine, Maywood, IL, USA
2Department of Neurology, Northwestern University, USA
3Lou & Jean Malnati Brain Tumor institute of the Robert H. Lurie Comprehensive Cancer Center, Chicago, IL, USA
4Department of Otolaryngology, Loyola University Medical Center/Stritch School of Medicine, Maywood, IL, USA
5Department of Neurological Surgery, Edward Hines, Jr. VA Hospital, Hines, IL, USA
6Department of Pathology, Loyola University Medical Center/Stritch School of Medicine, Maywood, IL, USA
7Department of Radiation Oncology, Loyola University Medical Center/Stritch School of Medicine, Maywood, IL, USA
8Department of Radiology, Loyola University Medical Center/Stritch School of Medicine, Maywood, IL, USA
9Department of Hemato-oncology, Loyola University Medical Center/Stritch School of Medicine, Maywood, IL, USA
10Department of Neurology, Loyola University Medical Center/Stritch School of Medicine, Maywood, IL, USA
*Corresponding author: Jigisha P Thakkar Loyola University Stritch School of Medicine, Department of Neurology and Neurosurgery, Division of Neuro-oncology, USA. Email: jigisha.thakkar@lumc.edu
Received: March 07, 2023 Accepted: April 22, 2023 Published: April 29, 2023
Abstract
We present three cases of O6-Methylguanine-DNA Methyltransferase (MGMT) methylated high grade gliomas with distant recurrence. All three patients had a radiographic stability of original tumor site at time of distant recurrence indicating impressive local control with Stupp protocol in patients with a MGMT methylated tumors. All patients had a poor outcome after distant recurrence. For one patient Next Generation Sequencing (NGS) was available for both original and recurrent tumor and did not reveal any difference other than high tumor mutational burden in the distant recurrent tumor. Understanding risk factors of distant recurrence in MGMT methylated tumors and investigating correlations between recurrences will help plan therapeutic strategies to prevent distant recurrence and improve survival of these patients.
Keywords: Distant recurrence; High grade glioma; MGMT methylated
Highlights
• Patients with positive MGMT promoter methylation status may have a higher rate of distant recurrence and survival is significantly shorter post-recurrence in patients with distal recurrence.
• The original tumor site was stable when distant recurrence occurred, indicating good local control with Stupp protocol in MGMT methylated tumors.
• The original tumor and distant tumor may harbor many of the same truncal mutations.
• However, the distant tumor may accumulate additional mutations which could be contributing factors to its spread.
Introduction
MGMT methylated high grade gliomas have better response to treatment and better survival as compared to MGMT unmethylated gliomas [1]. Distant recurrence is more common in MGMT methylated tumors [2]. We present a series of three cases of MGMT methylated gliomas with distant recurrence who had poor outcomes despite the MGMT methylated status. All these patients had stable original tumor site when distant recurrence occurred. This observation indicates good local control in MGMT methylated high grade glioma with current standard treatment. However, standard treatment fails to prevent distant recurrence and therapeutic strategies to prevent distant recurrence are warranted in selected MGMT methylated patients.
Cases
Patient 1
A sixty-five years old female was diagnosed with glioblastoma World Health Organization (WHO) grade 4, Isocitrate Dehydrogenase Enzyme (IDH) wild type (WT), MGMT methylated. She underwent complete resection of the left frontal tumor followed by treatment with Stupp protocol (concomitant temozolomide + radiation sixty gray x thirty fractions followed by six cycles of maintenance temozolomide. Postoperative ischemic volume was <1ml. Surveillance imaging one-year after initial surgery (four months post completion of Stupp protocol) revealed stable postoperative surgical cavity with development of a new remote rim-enhancing left temporal mass (Figure 1). She underwent re-resection with pathology revealing glioblastoma, IDH-WT, MGMT methylated with increased tumor mutational burden when compared with initial pathology. She was initiated on concurrent temozolomide and radiation for the recurrent tumor. However, her clinical course was complicated by perforated diverticulitis requiring hospitalization. She had significant deterioration in her functional status and the family opted to transition to hospice care (two months after second craniotomy).
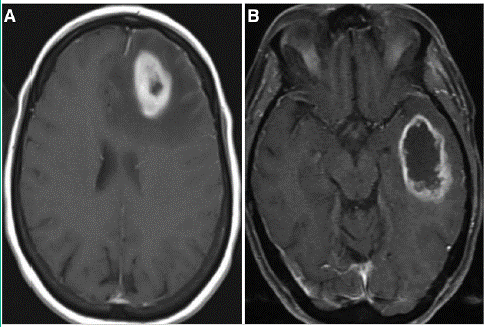
Figure 1: Axial view of magnetic resonance imaging of the brain with contrast. A: original tumor, ring enhancing lesion in left frontal lobe with surrounding edema and midline shift. B: distant new ring enhancing lesion in left temporal region.
This patient had distant recurrence four months after completion of Stupp protocol. The molecular profile (next generation sequencing by OncoPlus panel) of original and distant tumor was similar with exception of high tumor mutational burden in the recurrent tumor (Table 1). The original tumor site was stable when distant recurrence occurred indicating good local control with Stupp protocol.
Patient 2
A fifty-seven years old male with diagnosis of glioblastoma WHO grade 4, IDH WT, MGMT methylated. He underwent complete resection of right frontal tumor followed by treatment with Stupp protocol (concomitant temozolomide + radiation sixty gray x thirty fractions followed by six cycles of maintenance temozolomide. Postoperative ischemic volume was 18ml.
After completion of four cycles of temozolomide he had increased enhancement around the resection cavity. Resection of enhancing lesion revealed radiation necrosis. He then received six cycles of bevacizumab for radiation necrosis. Surveillance imaging sixteen months after initial surgery (five months post completion of Stupp protocol) revealed a stable postoperative surgical field but with development of a new remote rim-enhancing tumor in the posterior fossa (Figure 2).
Figure 2: Axial view of magnetic resonance imaging of the brain with contrast. A: Original tumor, ring enhancing lesion in right frontal lobe with surrounding edema and midline shift. B: Complete resection of right frontal tumor. C: Stable original right frontal tumor at the time of distant recurrence. D: Distant new enhancing lesion in left cerebellar tonsil and vermis.
This patient had distant recurrence five months after completion of Stupp protocol. The original tumor site was stable when distant recurrence occurred indicating good local control with Stupp protocol. The patient elected to be transitioned to hospice due clinical decline.
Patient 3
A thirty-one years old male patient diagnosed with IDH mutant, WHO grade 4, MGMT methylated astrocytoma. He underwent craniotomy with complete resection of left frontal tumor followed by treatment with Stupp protocol (concomitant temozolomide + radiation sixty grays x thirty fractions followed by six cycles of maintenance temozolomide. Postoperative ischemic volume was 2ml.
Surveillance imaging 21 months after initial surgery (approximately 10 months post completion of Stupp protocol) revealed stable postoperative surgical field and development of a new remote rim-enhancing tumor in the cerebellar vermis (Figure 3). A suboccipital craniectomy was performed with resection of vermian tumor and placement of ventriculoperitoneal shunt. He then received palliative radiation (twenty-five gray x five fractions). He had a significant decline in functional status and was transitioned to palliative care. This patient had distant recurrence ten months after completion of Stupp protocol. The original tumor site was stable when distant recurrence occurred indicating good local control with Stupp protocol.
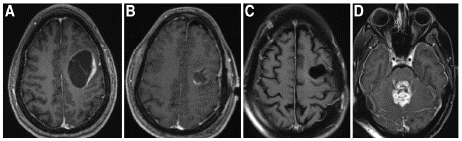
Figure 3: Axial view of magnetic resonance imaging of the brain with contrast. A: Original tumor, ring enhancing lesion in left frontal lobe with surrounding edema and midline shift. B: Complete resection of left frontal tumor. C: Stable original left frontal tumor at the time of distant recurrence. D: Distant new enhancing lesion in cerebellar vermis extending to the brain stem.
Review of Literature
Recurrence pattern in glioblastoma is classified into two simple groups; local recurrence and non-local/distant recurrence based on distance from original resection cavity and radiation field [2,3]. Local recurrence is more common and occurs early in the course of the disease [4-6]. Distant recurrence is less common and develops after a longer time interval as compared to local recurrence [7].
Distant recurrence is related to various factors including presence of MGMT promoter methylation Complete Resection of Enhancing Tumor (CRET), preoperative tumor volume (>50cc), post-surgical ischemia and tumor involvement of the Subventricular Zone (SVZ) [1,37-11]. Postoperative ischemic volume and hypoxia might introduce an infiltrative tumor growth with diffuse and more distant tumor recurrence patterns [11,12].
Patients with positive MGMT promoter methylation status have higher rate of distant recurrence which is likely due to better local control in these patients with temozolomide which may be a radiation sensitizer [2]. Temozolomide has systemic activity as well as higher response rates in MGMT methylated patients [1]. However, distant recurrence rate is higher in MGMT methylated patients. In our patient series of MGMT methylated high grade gliomas with distant recurrence, the original tumor site was stable at the time of distant recurrence indicating good local control with Stupp protocol. Survival is significantly shorter post-recurrence in patients with distal recurrence [3,12]. Similar trend of poor survival was seen in all three of our patients had poor outcomes after distant recurrence. Patients with positive MGMT methylation have significantly better survival. However, a minority of these patients develop distant recurrence which leads to poor prognosis despite the positive MGMT methylation status. The role of expanded field radiation in selected MGMT methylated patients to prevent distant recurrence should be explored in clinical trials.
Additionally, one would wonder if the genetic make-up of the distant tumor is different from the original tumor. Next generation sequencing for patient 1 revealed a both tumor samples were high tumor mutational burden in recurrent tumor which is likely temozolomide induced (Table 1). Both tumors were MGMT methylated. Understanding risk factors leading to distant recurrence and investigating correlations between recurrences will help plan therapeutic strategies to prevent distant failure and improve survival in high grade glioma patients.
Case 1: Comparison of molecular profile of original tumor and recurrent tumor
Molecular profile
Original Tumor
Distant recurrent tumor
IDH1/2 variants
Negative
Negative
EGFR amplification
Positive
Positive
TERT promoter variant
Positive
Positive
CDKN2A loss
Positive
Positive
Tumor Mutation burden
1.7 mutations/megabase
106.0 mutations/megabase
MGMT methylation
Positive
Positive
Abbreviations: IDH: Isocitrate Dehydrogenase Enzyme; MGMT: O6-Methylguanine-DNA Methyltransferase; EGFR: Epidermal Growth Factor Receptor; TERT: Telomerase Reverse Transcriptase; CDK: Cyclin-Dependent Kinase Inhibitor.
Table 1:
Author Statements
Disclosures
Jonathan Lee, Ignacio Jusue-Torres, Anand V. Germanwala, Ewa Borys, Abhishek A. Solanki, Atul K. Mallik,Kevin Barton, Jigisha P. Thakkar have no disclosures. Scientific Advosory Boards for Merck and Novocure, Speakers bureau for Merck and Novocure, and research support (drug only) from BMS. Honoraria for advisory board Monteris. Honoraria for medical editing Medlink Neurology, EBSCO Publishing.
References
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352: 997-1003.
- Milano MT, Okunieff P, Donatello RS, Mohile NA, Sul J, et al. Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys. 2010; 78: 1147-1155.
- Tejada S, Aldave G, Marigil M, Gallego Perez-Larraya J, Dominguez PD, et al. Factors associated with a higher rate of distant failure after primary treatment for glioblastoma. J Neurooncol. 2014; 116: 169-175.
- Liang BC, Thornton AF, Sandler HM, Greenberg HS. Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg. 1991; 75: 559-563.
- Gaspar LE, Fisher BJ, Macdonald DR, LeBer DV, Halperin EC, et al. Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992; 24: 55-57.
- Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989; 16: 1405-1409.
- Brandes AA, Tosoni A, Franceschi E, Sotti G, Frezza G, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol. 2009; 27: 1275-1279.
- Niyazi M, Schnell O, Suchorska B, Schwarz SB, Ganswindt U, et al. FET-PET assessed recurrence pattern after radio-chemotherapy in newly diagnosed patients with glioblastoma is influenced by MGMT methylation status. Radiother Oncol. 2012; 104: 78-82.
- De Bonis P, Anile C, Pompucci A, Fiorentino A, Balducci M, et al. The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg. 2013; 115: 37-43.
- Lim DA, Cha S, Mayo MC, Chen MH, Keles E, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007; 9: 424-429.
- Thiepold AL, Luger S, Wagner M, Filmann N, Ronellenfitsch MW, et al. Perioperative cerebral ischemia promote infiltrative recurrence in glioblastoma. Oncotarget. 2015; 6: 14537-14544.
- Bette S, Barz M, Huber T, Straube C, Schmidt-Graf F, et al. Retrospective Analysis of Radiological Recurrence Patterns in Glioblastoma, Their Prognostic Value And Association to Postoperative Infarct Volume. Sci Rep. 2018; 8: 4561.