
Case Report
Ann Hematol Onco. 2023; 10(3): 1424.
A Case of Autoimmune Metaplastic Atrophic Gastritis with Concurrent Pernicious Anemia and Coombs-Negative Hemolysis
Joseph P Marshalek*
Department of Internal Medicine, Harbor-UCLA Medical Center, USA
*Corresponding author: Joseph P Marshalek Department of Internal Medicine, Harbor-UCLA Medical Center, 1000 W Carson Street Torrance, CA, 90502, USA. Email: jmarshalek@dhs.lacounty.gov
Received: April 17, 2023 Accepted: May 13, 2023 Published: May 20, 2023
Abstract
Pernicious anemia is an autoimmune disease characterized by atrophic gastritis and vitamin B12 deficiency. Concurrent hemolytic anemia has been described, however its prevalence and mechanism are not well understood. Herein, we present the case of a 40-year-old female who presented with abdominal pain, fatigue, and a 50-lb weight loss over four months. She was found to have macrocytic anemia, leukopenia, moderate neutropenia, and severe vitamin B12 deficiency. Further laboratory testing (indirect hyperbilirubinemia, low haptoglobin, increased lactate dehydrogenase, low reticulocyte index) implied the presence of intravascular hemolysis with a poor bone marrow response. Peripheral blood smear showed hypersegmented neutrophils consistent with megaloblastic anemia and schistocytes suggestive of hemolysis. Notably, the direct Coombs test was negative.The patient’s serum was positive for anti-intrinsic factor and anti-parietal cell antibodies. Esophagogastroduodenoscopy revealed H. Pylori-negative, metaplastic atrophic gastritis with no evidence of malignancy. Thus, a diagnosis of pernicious anemia was confirmed in addition to hemolytic anemia. She was treated with intramuscular cyanocobalamin, resulting in improvement of symptoms, cytopenias, and hemolytic markers. Hemolysis is a rare complication of vitamin B12 deficiency, and multifactorial anemia must always be considered.
Keywords: Hemolytic anemia; Hemolysis; Autoimmune metaplastic atrophic gastritis; Pernicious anemia; Vitamin B12 deficiency
Abbreviations: G6PD: Glucose-6-Phosphate Dehydrogenase; LDH: Lactate Dehydrogenase
Introduction
Acquired hemolytic anemia is associated with a wide variety of clinical conditions and is mediated by immune and non-immune mechanisms. Immune-mediated mechanisms of hemolysis include warm and cold autoimmune hemolytic anemia, paroxysmal nocturnal hemoglobinuria, and most cases of drug-induced hemolytic anemia [1-4]. There is a vast array of non-immune causes of hemolytic anemia including thalassemia, sickle cell disease, G6PD deficiency, hereditary spherocytosis, infections, thrombotic microangiopathies, mechanical hemolysis from indwelling devices, hypersplenism, and various pregnancy syndromes [5-12].
Megaloblastic anemia due to vitamin B12-deficient autoimmune gastritis is referred to as pernicious anemia. Anti-intrinsic factor antibodies prevent the formation of the vitamin B12 – intrinsic factor complex which is required for vitamin B12 absorption. As a consequence, there is impaired hematopoiesis as vitamin B12 is a necessary co-factor in the folate-mediated synthesis of nucleic acids. The prevalence of autoimmune gastritis and pernicious anemia are roughly 2% and 0.4%, respectively [13,14].
While vitamin B12 deficiency and iron deficiency anemia are well-established sequelae of autoimmune metaplastic atrophic gastritis due to malabsorption, hemolytic anemia is a notably rare and not well understood complication [15,16]. In a cohort reported by Andres et al [16], the prevalence of hemolytic anemia in patients with vitamin B12 deficiency was 1.5%. However, there is a paucity of research regarding the prevalence and mechanism of concurrent pernicious anemia and hemolytic anemia. It is the aim of this case report to describe a case of severe autoimmune metaplastic atrophic gastritis with concomitant pernicious anemia and Coombs-negative hemolytic anemia.
Case Presentation
A 40-year-old female with Dandy-Walker malformation presented to the emergency room with epigastric pain, fatigue, and a 50-lb weight loss over the past four months. The epigastric pain was burning, non-radiating, and postprandial. On physical examination, the patient had conjunctival pallor and epigastric tenderness without rebound or guarding. No scleral icterus or jaundice was observed. She reported no glossitis, paresthesias, balance problems, or weakness. Sensory examination was normal including proprioception and vibration. Mental status examination was normal, and the patient denied any mood changes.
Complete blood count with differential showed macrocytic anemia (hemoglobin 7.3g/dL, mean corpuscular volume 110.1fL), leukopenia (white blood cell count 2400 per mm3) and moderate neutropenia (absolute neutrophil count 800 per mm³). Folic acid was elevated at 31.6ng/mL, and vitamin B12 was very low (<60pg/mL). Indirect hyperbilirubinemia (4.9mg/dL), low haptoglobin (<8mg/dL), and increased lactate dehydrodenase (LDH=1,204U/L) supported the presence of hemolysis. The reticulocyte index was 0.21, indicating a poor bone marrow response to hemolysis. Peripheral blood smear revealed hypersegmented neutrophils consistent with megaloblastic anemia and schistocytes compatible with hemolysis (Figure 1). Thus, it appeared the patient’s severe vitamin B12 deficiency was leading to both decreased erythrocyte production and increased erythrocyte destruction. Of note, the patient’s direct Coombs test was negative. The patient was started on intramuscular cyanocobalamin 1000mcg daily for vitamin B12 deficiency and given pantoprazole for gastrointestinal symptoms.
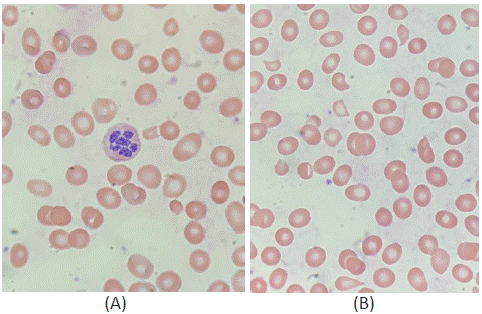
Figure 1: Peripheral blood smear showing: (A) Hypersegmented neutrophil; (B) Schistocytes and teardrop red blood cells.
Esophagogastroduodenoscopy was performed the following day. There was extensive loss of gastric rugae (Figure 2A), severe mucosal atrophy, and a 1cm antral hyperplastic polyp (Figure 2B). Gastric body biopsy revealed diffuse atrophy of the oxyntic mucosa with lack of oxyntic glands, focal pyloric metaplasia, and focal pancreatic metaplasia, which was consistent with metaplastic atrophic gastritis. Immunohistochemistry was negative for H. Pylori. The patient’s serum was positive for anti-intrinsic factor and anti-parietal cell antibodies. At this point, the patient was diagnosed with autoimmune gastritis and pernicious anemia.
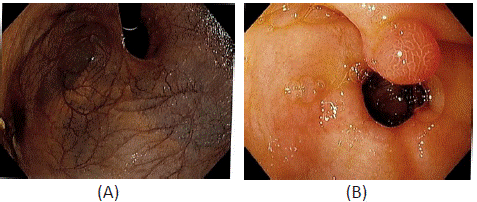
Figure 2: Esophagogastroduodenoscopy images showing: (A) Extensive loss of gastric rugae and severe mucosal atrophy with visible submucosal veins; (B) A 1cm gastric antral polyp.
Throughout the patient’s six-day hospital stay, she was treated with daily intramuscular cyanocobalamin and required one unit of packed red blood cells. The patient’s hemoglobin, white blood cell count, and absolute neutrophil count increased but remained below the normal range. She reported feeling much better after a few days, and hemolytic markers were improving. At discharge, the patient was instructed to take intramuscular vitamin B12 injections weekly for 1 month and then monthly there after.
Discussion
Autoimmune metaplastic atrophic gastritis is a potentially under-recognized condition which can cause severe gastrointestinal symptoms along with decreased vitamin B12 absorption and impaired hematopoiesis. In this case, impaired blood cell production manifested as megaloblastic anemia, leukopenia, and moderate neutropenia. Intriguingly, the case was further complicated by a Coombs-negative hemolytic process. Hemolysis is associated with a variety of inherited red blood cell disorders involving impairments in hemoglobin synthesis, erythrocyte membrane structure, and enzyme activity [5-7]. Therefore, it could be hypothesized that a patient with a deficiency of vitamin B12, which is an integral component of nucleotide synthesis and hematopoiesis, produces abnormal erythrocytes which are more susceptible to destruction via hemolysis.
The patient in this case report had peripheral blood smear anomalies and laboratory derangements consistent with both pernicious anemia and hemolytic anemia. The diagnosis of pernicious anemia was established with macrocytic anemia, severe vitamin B12 deficiency with a malabsorptive etiology (autoimmune gastritis), positive anti-intrinsic factor and anti-parietal cell antibodies, and hypersegmented neutrophils on peripheral blood smear. The presence of hemolysis was confirmed with low haptoglobin, elevated LDH, hyperbilirubinemia, and schistocytes on peripheral smear. A negative direct Coombs test seemed to be compatible with a non-immune mechanism of hemolysis.
Interestingly, treatment of the underlying vitamin B12 deficiency (via intramuscular cyanocobalamin to bypass the gastrointestinal absorptive impairment) was sufficient to stifle hemolysis, further supporting the hypothesis that disordered erythrocyte structure from vitamin B12 deficiency was the trigger for hemolysis, as opposed to two simultaneous unrelated phenomena. Continued research investigating the scope and mechanisms of acquired hemolytic anemia is imperative, and multifactorial anemia should always be considered.
Statements and Declarations
The author of this study has no financial or personal interests to disclose. This research did not receive any specific grant funding from agencies in the public, commercial, or not-for-profit sectors. Informed consent was obtained from the patient to publish this case report in accordance with the journal’s patient consent policy.
References
- Barellini W, Zaninoni A, Giannotta JA, Fattizzo, B. New insights in autoimmune hemolytic anemia: from pathogenesis to therapy. Journal of Clinical Medicine. 2020; 9: 3859.
- Colden MA, Kumar S, Munkhbileg B, Babushok DV. Insights into the emergence of paroxysmal nocturnal hemoglobinuria. Frontiers in Immunology. 2021; 12: 830172.
- Hwang SR, Saliba AN, Wolanskyj-Spinner AP. Immunotherapy-associated autoimmune hemolytic anemia. Hematology/ Oncology Clinics of North America. 2022; 36: 365-380.
- Mayer B, Bartolmas T, Yurek S, Salama A. Variability of findings in drug-induced immune haemolytic anaemia: experience over 20 years in a single centre. Transfusion Medicine and Hemotherapy. 2015; 42: 333-339.
- Nelwan EJ, Shakinah S, Pasaribu A. Association of G6PD status and haemolytic anaemia in patients receiving anti-malarial agents: a systematic review and meta-analysis. Malaria Journal. 2023; 22: 77.
- Lumbala PK, Mbayabo G, Ngole MN, Lumaka A, Race V, et al. Clinical and laboratory characterization of adult sickle cell anemia patients in Kinshasa. PLoS One. 2022; 17: e0278478.
- Zaninoni A, Fermo E, Vercellati C, Marcello AP, Barcellini W, et al. Congenital hemolytic anemias: is there a role for the immune system? Frontiers in Immunology. 2020; 11: 1309.
- Thompson GL, Kavanagh D. Diagnosis and treatment of thrombotic microangiopathy. International Journal of Laboratory Hematology. 2022; 44: 101-113.
- Almazan C, Scimeca RC, Reichard MV, Mosqueda J. Babesiosis and theileriosis in North America. Pathogens. 2022; 11: 168.
- Rodriguez FJ, Ritter MJ, Tauscher CD, Moore SB. Massive hemolysis secondary to alpha-toxin release. Transfusion. 2005; 45: 127.
- Lang E, Lang F. Triggers, inhibitors, mechanisms, and significance of eryptosis: the suicidal erythrocyte death. BioMed Research International. 2015; 2015: 513518.
- Gardikioti A, Venou TM, Gavriilaki E, Vetsiou E, Mavrikou I, et al. Molecular advances in preeclampsia and HELLP syndrome. International Journal of Molecular Sciences. 2022; 23: 3851.
- Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clinical Immunology and Immunopathology. 1997; 84: 223-243.
- Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. The American Journal of Clinical Nutrition. 1994; 60: 2-11.
- Oo TH, Rojas-Hernandez CM. Challenging clinical presentations of pernicious anemia. Discovery Medicine. 2017; 24: 107-115.
- Andres E, Affenberger S, Zimmer J, Vinzio S, Grosu D, et al. Current hematological findings in cobalamin deficiency: a study of 201 consecutive patients with documented cobalamin deficiency. Clinical and Laboratory Haemotology. 2006; 28: 50-56.