
Case Report
Ann Hematol Onco. 2023; 10(3): 1425.
Radiotherapy for Myeloid Sarcoma of the Breast: A Case Report and Review of the Literature
Dortmans J*; Zissiadis Y
Department of Radiation Oncology, Genesis Care, Fiona Stanley Hospital, 11 Robin Warren Dr, Murdoch WA 6150 Australia
*Corresponding author: Dortmans J Department of Radiation Oncology, Fiona Stanley Hospital, 11 Robin Warren Dr, Murdoch WA 6150, Australia. Tel: (+61) 86152 2222 Email: jessica.dortmans@health.nsw.gov.au
Received: April 20, 2023 Accepted: May 15, 2023 Published: May 22, 2023
Abstract
While the role of systemic therapy in the treatment of myeloid sarcoma is well documented, the evidence for the use of radiation therapy is sparse. We present a case of isolated myeloid sarcoma to the breast. Following treatment for myelodysplastic syndrome. Myeloid Sarcomas (MS) are also known as chloromas and granulocytic sarcomas. They are extramedullary tumours of immature myeloid cells that develop in the setting of Acute Myeloid Leukaemia (AML), Chronic Myeloid Leukaemia (CML) and accelerated phase MyeloDysplastic Syndrome (MDS) [1]. Treatment options include systemic therapy, localised radiation treatment or surgery. Retrospective series have identified that radiotherapy provides safe and reliable local control and symptom palliation [2]. We conducted a review of literature to assess the rationale, role, and dose of radiotherapy for this rare leukemia manifestation in the breast.
Keywords: Myeloid sarcoma; Chloroma; Granulocytic sarcoma; Breast; Radiotherapy
Methods
A literature search was performed in MEDLINE and Pubmed for articles on myeloid sarcoma to the breast published between 1972 and August, 2020 using the following keywords and phrases: myeloid sarcoma, chloroma, granulocytic sarcoma, extramedullary sarcoma, breast, radiotherapy, radiation therapy. Additional evaluated articles were identified by back referencing from bibliographies of original articles. The search was limited to English language articles.
Case Presentation
We present a case of a 52-year-old woman with myeloid sarcoma of the left breast and axillary nodes following allogenic matched unrelated bone marrow transplant for Myelodysplastic Syndrome (MDS) two years prior.
This patient was initially diagnosed with MDS, treated with azacitidine followed by matched unrelated bone marrow transplant 10 months following diagnosis.
20 months following her transplant she self-detected a left breast mass, evident on mammogram which confirmed a 28mm dense mass in the upper outer quadrant. Ultrasound showed a 30mm poorly defined mass with heterogenous echotexture, and enlarged left axillary lymph nodes.
Core biopsy of breast lesion showed Myeloid Sarcoma (MS), with mixed phenotype. Bone marrow aspirate and trephine revealed slightly hypercellular marrow with reactive changes, showing no evidence of excess blasts and the chimerism was 99% donor. There was no evidence of systemic relapse. Staging PET scan showed a solitary FDG-avid left breast lesion and two ipsilateral axillary lymph nodes. No abnormal FDG avidity was seen elsewhere to suggest metastases.
Treatment options presented to the patient included systemic therapy, localised radiation treatment or close surveillance. The patient opted to proceed with localised involved field radiation therapy, with the aim of locoregional control and delaying time to systemic therapy, accepting that this treatment approach was unlikely to result in cure.
Radiotherapy was delivered using a Deep Inspiration Breath Hold technique (DIBH) to minimise cardiac dose with a left sided breast malignancy, and utilised IMRT treatment to deliver a dose of 40Gy in 20 fractions over 4 weeks. The treatment volume included the entire left breast, and left axilla. Coverage of >95% was achieved for all volumes.
The patient tolerated treatment well, with a good clinical response in both the breast and axilla at completion of treatment.
Reference
Study
Level of evidence
Presentation
Clinical picture
Systemic disease
Treatment
Radiotherapy
Outcome
[8]
Barloon et al, 1993
Case report
Palpable breast masses
Isolated relapse post treatment for AML
No
Radiotherapy alone
24Gy/10#
No evidence of local disease at 10 weeks
[12]
Breccia et al, 1995
Case report
Palpable breast and ipsilateral neck masses
Primary localised myeloid sarcoma
No
Radiotherapy, chemotherapy
36Gy/18#
No clinical evidence of disease post radiotherapy. Disease free at 19 months
[13]
Au et al,1999
Case report
Palpable slow growing unilateral breast mass
Isolated relapse post multiple replapses of AML
No
Chemotherapy, radiotherapy, autologous SCT
30Gy
No clinical response in breast, progressive disease at 4 months
[14]
Guermazi et al, 2000
Case report
Palpable unilateral breast mass
Isolated relapse post treatment for AML
No
Chemotherapy
-
No clinical evidence of local disease at 5 weeks
[7]
Quintas-Cardama et al, 2003
Case report
Palpable right breast mass
Primary unilateral breast MS
No
Lumpectomy, chemotherapy, consolidative radiotherapy
36Gy/18#
Disease free at 37 months
[15]
Shea et al, 2004
Case report
Screening detected bilateral breast masses
Primary bilateral breast MS
No
Chemotherapy, autologous SCT, radiotherapy to bilateral breasts
Dose not reported
Disease free at 24 months
[16]
Roy et al, 2004
Case report
Palpable left breast mass
Primary unilateral breast MS
No
Biopsy, chemotherapy
-
-
[5]
Fu et al, 2014
Case reports
Clinical left breast mass
Primary MS
No
Lumpectomy + chemotherapy
-
Disease free at 4 years
Clinical left breast mass
Relapsed AML with MS to breast
Unknown
Lumpectomy + chemotherapy
-
Overall survival 38 months
[17]
Gunduz et al, 2014
Case report
Palpable right breast mass
Isolated relapse post treatment for AML
No
Chemotherapy, autologous SCT
-
Deceased 24 days post chemotherapy due to sepsis
[18]
Goncalves et al, 2014
Case report
Palpable left breast mass
Primary unilateral breast MS
No
Lumpectomy, chemotherapy, radiotherapy
30Gy/15#
Disease free at 26 months
[19]
Huang et al, 2015
Case report
Clinical mass left breast
AML treated with chemotherapy, solitary relapse to breast
No
Lumpectomy, Chemotherapy
-
Local control at 1 year
[20]
Nalwa et al, 2015
Case report
Clinical right breast mass
Primary bilateral breast MS
No
Mastectomy + axillary lymph node dissection, chemotherapy
-
Disease free at 12 months
[21]
Gomaa et al, 2018
Case report
Clinical left breast mass
Primary MS breast
No
Chemotherapy, radiotherapy
Dose not reported
No systemic disease at 6 months
[22]
Sharma et al, 2018
Case report
Palpable right breast mass
Primary unilateral MS
No
Lumpectomy, chemotherapy
-
Disease free at 12 months
[4]
Dominguez Rullán et al, 2018
Case report
Asymptomatic chest mass
Isolated myeloid sarcoma
No
Local excision, radiotherapy, chemotherapy
40Gy/20#
Progression to AML
[23]
Bubulac et al, 2019
Case report
Ultrasound detected breast masses
AML with MS relapses to breast x3
No
Chemotherapy, allogenic SCT, chemotherapy for relapses x2, last relapse treated with radiotherapy
Dose not reported
OS 36 months
[24]
Wu et al, 2019
Case report
Palpable right breast mass
Primary unilateral breast MS
No
Mastectomy, sentinel lymph node biopsy, chemotherapy
-
Disease free at 1 year
[25]
Minoia 2019
Case report
Clinical left breast mass
Primary bilateral breast MS
No
Chemotherapy, radiotherapy
30Gy/15#
Disease free at 27 months
She suffered only a mild skin reaction with intact skin and grade 1 erythema.
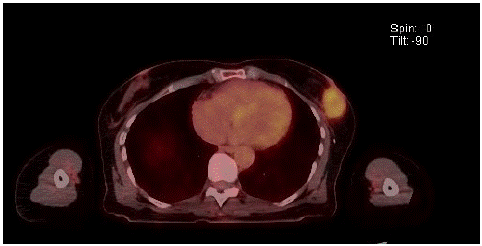
Figure 1: FDG PET showing MS left breast mass.
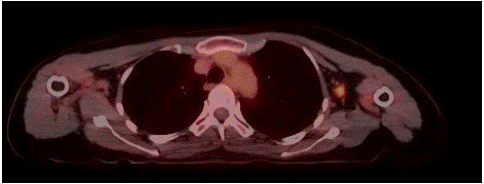
Figure 2: FDG PET showing MS in axillary lymph node.
At clinical follow up 6 weeks post completion of radiotherapy the patient had a significant clinical response, with decrease in size of the breast mass and no palpable axillary adenopathy. Her post-treatment PET scan obtained 10 weeks post completion of radiotherapy showed a complete metabolic response in both the left breast and axilla, with an absence of any distant disease evident.
Discussion
Haematological malignancies may manifest as extramedullary soft tissue masses known as myeloid sarcomas, granulocytic sarcomas, extramedullary sarcomas or chloromas and are a rare extramedullary tumour of immature myeloid cells [3]. These may occur in isolation, or more commonly in patients with a history of Acute Myeloid Leukemia (AML), MDS, or in blast phase of Chronic Myeloid Leukemia (CML) [4].
The reported cases of myeloid sarcomas localised to the breast are scarce in the literature [5]. In a series of 96 cases of MS reported by Mayo Clinic, only 3% of cases were within the breast [6]. Cases have been treated with chemotherapy, autologous stem cell transplant, surgery and radiotherapy.
Treatment strategies include systemic therapy while Radiotherapy (RT) and surgery have been used to improve local control [4]. RT alone is effective for local control but unlikely to provide long term disease control or cure. Systemic chemotherapy is recommended to achieve long term remission or, in some cases, to prevent progression to AML [7]. While it is uncommonly treated with radiotherapy alone, there are reports of low dose radiotherapy achieving complete mammographic response of isolated MS to the breast [8].
With local therapy alone, progression usually occurs within 10–12 months after the diagnosis, suggesting that isolated MS should be considered as a systemic disease and initial treatment should include chemotherapy with or without transplantation [4,9]. A retrospective study from 1986 showed that 25% of patients initially treated without systemic therapy did not progress to haematological diseases during follow-up ranging from 3.5–16 years [10]. Collated retrospective data demonstrate that 88–100% of patients progressed to AML with exclusive local therapy, compared to 42% when given systemic chemotherapy [11].
A review of the published case reports specifically for breast myeloid sarcoma without systemic leukaemia or myelodysplastic syndrome, their presentation, treatment and radiotherapy doses (if reported) are summarised below.
The role of RT in the treatment of MS has not been well established. Overall, RT without systemic treatment is not considered an optimal therapy for primary MS patients. It can be used in conjunction with systemic therapies, primarily in patients who need rapid relief of symptoms, or as a consolidation therapy [26]. It has been suggested that radiotherapy may prolong failure free survival but not overall survival in patients presenting with isolated MS. This study also included one case of MS successfully treated with RT alone with 12 months failure-free-survival [27]. A series of 21 patients with isolated MS demonstrated that disease recurrence was lower in a group that received chemoradiotherapy than those receiving radiotherapy alone, with local control rates of 97% [2]. Small, retrospective reviews where radiotherapy is used in combination with chemotherapy have failed to show an overall survival advantage, and did not report on failure-free survival or local control [28,29]. Due to the rarity of this diagnosis, there are no randomised studies of radiation therapy for MS. Many retrospective studies only report the use of radiotherapy for MS in the urgent setting – progressive neurological failure in a patient with spinal MS, and superior vena cava syndrome in a patient with cardiac MS [30].
Radiotherapy should be considered in isolated MS, inadequate response to chemotherapeutic regimen, in recurrence following bone marrow transplantation, and when rapid symptom relief is needed. Additionally, radiation provides excellent palliation and is recommended when symptom relief is required [2].
There are few studies that have addressed the role of radiotherapy or defined an appropriate dose. When RT is used following inadequate response to chemotherapy, and for palliation in circumstances that require rapid symptom relief a low dose regimen of 24Gy in 12 fractions can be used for most patients and produces excellent disease control and minimal morbidity [2]. One retrospective case review of 20 patients with 43 separate radiotherapy courses identified a 65% completed response rate, 25% with partial response, and 10% with stable disease. Doses ranged from 6-35Gy, with a median of 20Gy. 95% of these patients achieved symptomatic relief of MS after RT [31]. Another retrospective review of 41 patients treated with radiotherapy for MS with a median dose of 24Gy (range 5–36Gy), report a local control rate of 93% [1]. Lower dose ranges from have been reported to provide symptomatic relief and minimise disease burden if a more protracted course of treatment is not feasible [3].
Of the available published evidence on breast MS radiotherapy doses, when reported, range from 24-40Gy [4,7,8,12,13,18,25] and majority of these treatments were in conjunction with chemotherapy. Our dose of 40Gy in 20 fractions was selected as a monotherapy for this patient with the aim of providing durable local control and delaying systemic therapy. MS is considered radiosensitive and has been shown to have a dose-response relationship. The complete response rate in relation to dose has been reported as 43% with doses of 10–19.99Gy, 86% with doses of 20–29.99Gy and 89% with doses above 30Gy [32].
While the treatment techniques for MS have been sparsely reported, there is documented successful use of electrons, photons including three-dimensional conformal techniques, intensity modulated RT which may be beneficial in treating head and neck areas, and adaptive radiotherapy [3,33]. Margins from 0.5cm to 3cm have been recommended [3,4,31]. Again, given the absence of systemic therapy treatment for this patient at this time, we opted for margins at the upper end of reported literature. Once a 3cm margin had been added, majority of the left breast tissue was within the clinical tumour volume to be covered, so a decision to treat the entire left breast was made. A deep inspiration breath hold technique was utilised due to the robust evidence in the primary breast cancer setting that this significantly decreases cardiac dose [34].
Isolated myeloid sarcoma is rare, and treatment modalities include surgical excision, RT and chemotherapy. Radiotherapy can be considered when MS presents isolated, when there is partial response to chemotherapy, or as a palliative measure to rapidly relive symptoms. The proposed doses for isolated breast lesions range from 24-40Gy, however there is a significant dose-response correlation. These doses have a high likelihood of achieving local disease control with minimal morbidity. Radiotherapy may improve disease free survival or progression free survival, but due to a paucity of cases and a lack of randomised data this currently remains unproven.
References
- Hall MD, Chen YJ, Schultheiss TE, Pezner RD, Stein AS, et al. Treatment outcomes for patients with chloroma receiving radiation therapy. Journal of Medical Imaging and Radiation Oncology. 2014; 58: 523–7.
- Bakst R, Wolden S, Yahalom J. Radiation Therapy for Chloroma (Granulocytic Sarcoma). Int J Radiat Oncol Biol Phys. 2012; 82: 1816–22.
- Bakst RL, Dabaja BS, Specht LK, Yahalom J. Use of radiation in extramedullary leukemia/chloroma: guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018; 102: 314-9.
- Dominguez Rullán JA, Fernández Lizarbe E, Capuz B, Piris M, Sancho García S. Radiotherapy for isolated granulocytic sarcoma: Case report and review of literature. Clinical and translational radiation oncology. 2018; 8: 1-3.
- Fu J, Luo J. Granulocytic sarcoma of the breast in acute myeloid leukemia: Two case reports. Oncol Lett. 2014; 7: 145–7.
- Yui J, Mudireddy M, Patnaik M, Gangat N, Ak-Kali A, et al. Myeloid Sarcoma: The Mayo Clinic Experience of Ninety Six Case Series. Blood. 2016; 128: 2798.
- Quintas-Cardama A, Fraga M, Antunez J, Forteza J. Primary extramedullary myeloid tumor of the breast: a case report and review of the literature. Ann Hematol. 2003; 82: 431-4.
- Barloon T, Young DC, Bass SH. Multicentric granulocytoc sarcoma (chloroma) of the breast: Mammographic findings. American Journal of Roentgenology. 1993; 161: 963-4.
- Almond LM, Charalampakis M, Ford SJ, Gourevitch D, Desai A. Myeloid Sarcoma: Presentation, Diagnosis, and Treatment. Clinical Lymphoma Myeloma and Leukemia. 2017; 17: 263-7.
- Meis JM, Butler J, Osborne BM, Manning JT. Granulocytic Sarcoma in Nonleukemic Patients. Cancer. 1986; 58: 2697-709.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Therapeutic Advances in Hematology. 2011; 2: 309-16.
- Breccia M, Petti MC, Fraternali-Orconi G, Monarca B, Latagliata R, et al. Granulocytic sarcoma with breast and skin presentation: a report of a case successfully treated by local radiation and systemic chemotherapy. Acta Haematologica. 2000; 104: 34-7.
- Au WY, Ma SK, Kwong YL, Lie AKW, Shek WH, et al. Acute myeloid leukemia relapsing as gynecomastia. Leuk Lymphoma. 1999; 36: 191–4.
- Guermazi A, Quoc SN, Socie G, Briere J, de Kerviler E, et al. Myeloblastoma (chloroma) in leukemia: case 1. Granulocytic sarcoma (chloroma) of the breast. J Clin Oncol. 2000; 18: 3993-6.
- Shea B, Reddy V, Abbitt P, Benda R, Douglas V, Wingardet al. Granulocytic Sarcoma (Chloroma) of the Breast: A Diagnostic Dilemma and Review of the Literature. Breast J. 2004; 10: 48-53.
- Roy S, Staffod JS, Scally J, Selvachandran SN. Granulocytic sarcoma of the breast antedating acute myelogenous leukemia. The Breast. 2004; 13: 242-6.
- Gündüz E, Akay MO, Karagülle M, Ak IS. Isolated Granulocytic Sarcoma of the Breast after Allogeneic Stem Cell Transplantation: A Rare Involvement Also Detected by 18FDG-PET/CT. Turk J Haematol. 2014; 31: 88-91.
- Gonçalves J, Vasco Louro L, Ribeiro I, Oliveira A, Castro C. Radiotherapy for granulocytic sarcoma of the breast—Case report and review of the literature. Reports of practical oncology and radiotherapy. 2014; 19: 343–6.
- Huang XE, Li YJ, Zhou XD. Granulocytic sarcoma of the breast: A case report. Oncol Lett. 2015; 10: 2447-9.
- Nalwa A, Nath D, Suri V, Jalamuddin A, Srivastava A. Myeloid sarcoma of the breast in an aleukemic patient: a rare entity in an uncommon location. Malaysian J Pathol. 2015; 37: 63–6.
- Gomaa W, Ghanim A, Emam E, Bayoumi K, Ghanim A. Primary Myeloid Sarcoma of the Breast: A Case Report and Review of Literature. J Microsc Ultrastruct. 2018; 6: 212–4.
- Sharma A, Das AK, Pal S, Bhattacharyya S. Fine-needle aspiration cytology of granulocytic sarcoma presenting as a breast lump – Report of a rare case with a comprehensive literature search. J Lab Physicians. 2018; 10: 113-5.
- Bubulac L, Bardas A, Popa DC, Vasilache ED, Ionescu BO, et al. Breast myeloid sarcoma after allogeneic stem cell transplantation for acute myelomonocytic leukemia - case report. Romanian journal of morphology and embryology. 2019; 60: 707-11.
- Wu HY, Liu L, Gu L, Luo YH. Clinical characteristics and management of primary granulocytic sarcoma of the breast. Medicine (Baltimore). 2019; 98: e16648.
- Minoia C, de Fazio V, Scognamillo G, Scattone A, Maggialetti N, et al. Long-Lasting Remission in De Novo Breast Myeloid Sarcoma Treated with Decitabine and Radiotherapy. Diagnostics (Basel). 2019; 9: 84.
- Yilmaz AF, Saydam G, Sahin F, Baran Y. Granulocytic sarcoma: a systematic review. Am J Blood Res. 2013; 3: 265-70.
- Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003; 17: 1100-1103.
- Avni B, Rund D, Levin M, Grisariu S, Ben-Yehuda D, et al. Clinical implications of acute myeloid leukemia presenting as myeloid sarcoma. Hematol Oncol. 2012; 30: 34-40.
- Lan TY, Lin DT, Tien HF, Yang RS, Chen CY, et al. Prognostic factors of treatment outcomes in patients with granulocytic sarcoma. Acta Haematol. 2009; 122: 238-46.
- Antic D, Elezovic I, Milic N, Suvajdzic N, Vidovic A, et al. Is there a “gold” standard treatment for patients with isolated myeloid sarcoma?. 2013; 67: 72-7.
- Chen W, Wang C, Chang C, Liu H, Lan K, et al. Clinicopathologic features and responses to radiotherapy of myeloid sarcoma. Radiat Oncol. 2013; 8: 245.
- Chak LY, Sapoz Ink, Cox RS. Extramedullary lesions in non-lymphocytic leukemia: results of radiation therapy. Int J Radiat Oncol Biol Phys. 1983; 9: 1173-6.
- Majdoul S, Colson-Durand L, To NH, Belkacemi Y. Adaptive Radiotherapy for an Uncommon Chloroma. Case Rep Oncol. 2016; 9: 593-8.
- Smyth L, Knight K, Aarons Y, Wasiak J. The cardiac dose-sparing benefits of deep inspiration breath-hold in left breast irradiation: a systematic review. J Med Radiat Sci. 2015; 62: 66-73.