
Research Article
Ann Hematol Onco. 2023; 10(3): 1426.
DNA Mismatch Repair Deficiency in Colorectal Adenocarcinoma by a Two-Antibody Immunohistochemical Approach and its Association with Clinicopathological Features among Bangladeshi Patients
Shiraj-Um-Mahmuda S¹; Begum F²; Rahman MM³; Islam T4; Shabnam US5; Afroz S6; Emita U7; Islam KBMS8*
1Lecturer, Department of Pathology, Dhaka Medical College, Bangladesh
2Professor, Department of Pathology, Faculty of Basic Science, Bangabandhu Sheikh Mujib Medical University, Bangladesh
3Associate Professor, Department of Pathology, Faculty of Basic Science, Bangabandhu Sheikh Mujib Medical University, Bangladesh
4Specialist (Pathology), Square Hospital, Bangladesh
5Medical officer, Department of Pathology and Microbiology, National Institute of Diseases of the Chest and Hospital, Bangladesh
6Medical officer, Department of Histology, National Institute of Cancer Research and Hospital, Bangladesh
7Clinical Pathologist, Khulna Medical College Hospital, Bangladesh
8Professor, Department of Medicine and Public Health, Faculty of Animal Science and Veterinary Medicine, Sher-e-Bangla Agricultural University, Bangladesh
*Corresponding author: KBM Saiful Islam Department of Medicine and Public Health, Faculty of Animal Science and Veterinary Medicine, Sher-e-Bangla Agricultural University, Dhaka-1207, Bangladesh. Tel: +8802-44814101 Email: vetkbm.meph@sau.edu.bd
Received: April 21, 2023 Accepted: May 15, 2023 Published: May 22, 2023
Abstract
Background: Defective DNA Mismatch Repair (dMMR) genes cause dMMR/Microsatellite Instability (MSI)-related Colorectal Cancers (CRC) in humans which are different from Microsatellite Stable (MSS) tumors in terms of biological behavior, therapeutic response, and prognosis. We aimed to determine the frequency of dMMR CRCs by a two-antibody (PMS2 and MSH6) immunohistochemical approach and to evaluate their association with clinicopathological parameters to document the ever first report of such cases in Bangladesh.
Methods: Fifty histopathologically confirmed resected bowel specimens of CRC were studied over a period of two years at a tertiary-level hospital in Bangladesh. Histopathological parameters like morphologic variants, histologic subtypes, grade, stage, Lymphovascular Invasion (LVI), intratumoral lymphocytic infiltrate, and Crohn-like peritumoral reaction were assessed. Immunohistochemistry using PMS2 and MSH6 was performed on representative paraffin blocks by DAKO EnVision method. Expression of both of the markers was evaluated in classified groups.
Results: Mean age of study population was 48.60±14.6 years with a male to female ratio of 1.8:1. dMMR was recorded in 32% of cases. Expression of PMS2 and MSH6 were lost in 20% and 12% of cases, respectively. dMMR status was significantly associated with mucinous histology (p=0.014), lower pN staging (p=0.042), low LVI (p=0.002), exhibited intra-tumoral lymphocytosis (p=0.001), and Crohn like peritumoral reaction (p=0.001). No significant association with gender, age, right-sided location, histologic type, pT stage or grade was observed.
Conclusion: Frequency of dMMR CRCs was comparatively higher in the Bangladeshi population than in other races. Identification of dMMR tumors by their protein expression pattern using at least two, preferably four antibodies is proposed for routine screening of CRC cases.
Keywords: Mismatch repair deficiency; Microsatellite instability; Colon; Immunohistochemistry; PMS2; MSH6 etc.
• Key points: Around one third CRC cases of Bangladesh are dMMR/MSI-related CRCs
• dMMR/MSI-related CRCs are more prevalent in Bangladeshi male patients below 50 years of age than that in female.
Introduction
Colorectal Cancer (CRC) is one of the most common cancers affecting humans globally. It is the second most common malignancy in women and the third most common malignancy in men counting 9.4% and 10.6% of all cancer cases, respectively [1]. The global burden of colorectal cancer is expected to increase by 60% by 2030. Its incidence shows a 10-fold variation across the world [2]. The prevalence of colorectal cancer is lower in Asia than in Western countries. But the incidence has been alarmingly increasing in countries of Asia-Pacific region during the last two decades due to the westernization of lifestyles [3]. In Bangladesh 5-year prevalence of colon and rectal cancer are 3.28 and 3.1 per 100,000 populations respectively [1]. CRCs develop through a series of events leading to the transformation of normal mucosa to adenoma and then to carcinoma. Three distinct molecular pathways of colorectal carcinogenesis including Chromosomal Instability (CIN), Microsatellite Instability (MSI) and CpG Island Methylation (CIMP) have been recognized with overlap between these pathways [4]. Microsatellite instability has been detected in 15% and 90% of cases of sporadic CRC and CRC secondary to Hereditary Non-Polyposis Colorectal Cancer (HNPCC), respectively [5].
The DNA replication process is not error-free. DNA Mismatch Repair System (MMR) is the cellular post-replication process that preserves DNA homeostasis and guarantees of genomic stability [6]. At least five different MMR proteins including MSH2, MLH1, PMS1, MSH6, and PMS2 are required to perform DNA mismatch repair [7]. Any inherited or somatic mutation or epigenetic silencing of any of these genes lead to MSI and the tumors associated with this are referred to as MSI high or MSI-H tumors [8].
Clinicopathologic presentation, biological behavior, treatment options, therapeutic response and prognosis of MSI-H colorectal cancers show some differences from Microsatellite Stable (MSS) tumors of the same stage [9-11]. There are two methods for screening of MSI/dMMR cases. One is to detect the amplified microsatellite loci by PCR and another is the detection of proteins encoded by DNA Mismatch Repair Genes (MMR) including MLH1, PMS2, MSH2 and MSH6 by Immunohistochemistry (IHC). IHC is a specific, sensitive, fast and cost-effective tool for detecting MSI/dMMR colon cancers. The predictive value of IHC using all four antibodies is virtually equivalent to that of MSI testing by PCR [12].
Mismatch repair proteins form functional heterodimer complexes during repair, MLH1 with PMS2, (MutLa heterodimer) and MSH2 with MSH6 (MutS a heterodimer). MLH1 and MSH2 are the obligatory partners which stabilize the secondary partners PMS2 and MSH6, respectively to protect from proteolytic degradation. As a result, loss of the MLH1 protein leads to PMS2 degradation while loss of MSH2 leads to loss of MSH6. However, the converse is not true because the obligatory partners can bind with other minor proteins. Based on this concept, a “two-stain” method using only the MSH6 and PMS2 proteins has been developed and employed by several studies that demonstrated this 2-antibody approach is as effective as using the 4-antibody panel with the further reduction of time and resources [13-16].
The detection of dMMR status is becoming more and more important for patients’ survival because of its crucial therapeutic, prognostic and predictive implications. In another study, we documented an increased trend towards young age Colorectal Carcinoma (CRC) in the Bangladeshi population over recent years [17]. However, neither the dMMR status in Bangladeshi CRC patients is documented nor their morphological features are studied in the Bangladeshi population yet. Therefore, the study aimed to determine the frequency of dMMR CRC cases by a two-antibody immunohistochemical approach and to evaluate their association with several clinical and histopathological parameters to document the ever first report of such cases in Bangladesh.
Methodology
Ethical Approval
Advanced approval was obtained from the local Ethics Committee (Institutional Review Board) of Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh for the study. All participants were informed about the nature & purpose of the study and prior written consent was obtained.
Exclusion Criteria
Clinically suspected colorectal carcinoma subsequently proved to be non-epithelial tumors of the colon were excluded from this study. Patients with a history of pre-operative chemo and/or radiation therapy, tumors composed mostly of mucin and a very small number of cells, cases with lost expression of immunomarkers in both internal control and tumor cells were also excluded from the study.
Study Design, Period and Sample
A cross-sectional, descriptive, hospital-based, study was conducted at the Department of Pathology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh for a period of two years (from March 2019 to February 2021). A total of 50 Paraffin blocks of large bowel resection specimens histologically diagnosed as adenocarcinoma were taken as the samples for the study. All the slides of the study cases were retrieved and reviewed. Then, representative paraffin-fixed tissue blocks were selected that showed both tumor and adjacent non-neoplastic control tissue. Demographic and clinical information was obtained from patients’ attendants using a pre-tested questionnaire.
Pathological Analysis
Selected cases were evaluated elaborately and parameters including gross feature, histological type, tumor grading, staging, lymphovascular invasion, Crohn’s-like peri-tumoral reaction and intratumoral lymphocytic infiltrate were assessed. One representative section from each case was selected for immunohistochemical staining with PMS2 and MSH6.
Histopathological Features
Selected Hematoxylin and Eosin (H&E) slides cases were independently reviewed by two accredited histopathologists of BSMMU. Evaluation of tumor features and host response were done using the following criteria:
Tumor Features
Mucinous histology: Extracellular mucin accumulation bounded either by tumor epithelium or stroma. Tumors were sub-grouped as mucinous histology being none, 1–50%, and >50% of tumor area involved [18].
Signet ring differentiation: Presence of tumor cells with intracytoplasmic mucin and peripherally displaced crescent-shaped nucleus, whether present within extracellular mucin pools or invading the stroma. These tumors were subcategorized by- no signet ring cell, signet ring cell involving 1-50% and >50% of the tumor area [19].
Medullary pattern: Sheets, trabeculae, or nests of small to medium-sized tumor cells showing syncytial pattern, frequent mitosis, and abundant stromal lymphocytic infiltration.
Features of the Host’s Immune Response
Crohn-like peri-tumoral reaction: characterized by the pronounced lymphoid reaction to tumor, composed of lymphoid follicles at tumor edges, not associated with either mucosa or pre-existing lymph node. Two or more large lymphoid aggregates in a section were required for the presence of this feature [20].
Intra-tumoral lymphocytic infiltrate: marked by the presence of small round lymphocytes within neoplastic epithelial cells. Subgrouping of this category was done into none, mild to moderate (up to two Intra-Epithelial Lymphocytes (IEL)/HPF) and marked (≥ 3 IEL/HPF) by a semi-quantitative method [21].
Immunohistochemical Study
Immunohistochemical study was performed by a two-antibody panel of MMR proteins containing MSH6 and PMS2 using the DAKO EnVision method on the representative paraffin-fixed tissue blocks. Three to four μm thick tissue sections were deparaffinized in xylene, rehydrated in alcohol, and washed in distilled water. All the antibodies were ready-to-use monoclonal antibodies provided in liquid form in a buffer containing stabilizing protein and 0.015 mol/L sodium azide (PMS2 clone, EP51; MSH6 clone, EP49). The formalin-fixed, paraffin-embedded tissue sections were pretreated with heat-induced epitope retrieval (HIER) at 97°C for 35–40 min at high pH (50×). The slides were then incubated with PMS2 and MSH6 antibodies. Normal/intact staining pattern was defined as the presence of unequivocal nuclear staining (staining intensity at least similar to control) in any percentage of malignant cells, while nuclear staining in adjacent non-neoplastic tissue (lymphocytes, basal colonic crypt cells, and some stromal cells) was considered as a positive internal control. Negative staining was defined as the complete absence of nuclear staining in malignant cells where internal control was positive. Hence, carcinoma was considered dMMR when there was the absence of nuclear staining for at least one of the selected proteins. Tumors in which internal control and tumor cells both fail to express the markers were excluded from the study.
• MMR status was assigned on the basis of IHC testing as below:Deficient MMR (dMMR): Cases showing absence of detectable staining in 100% tumor nuclei with one or both of the IHC markers tested.
• Proficient MMR (pMMR): Normal expression of both markers in any percentage of tumor nuclei detected by immunohistochemistry.
Statistical Analysis
The statistical analysis was carried out using the Statistical Package for Social Sciences version 22.0 for Windows (SPSS Inc., Chicago, Illinois, USA). The result was calculated by using descriptive statistical formulas and presented in Tables, Figures, and Diagrams. The frequency of different entities was expressed as percentage. The association of expressions of selected markers with clinicopathologic parameters was evaluated with unpaired T-Test and Fisher’s exact test.
Results
Out of total 50 CRC cases, 32% (n=16) cases showed loss of expression of at least one MMR protein (dMMR). Expressions of PMS2 and MSH6 proteins were lost in 20% (n=10) cases and 12% (n=6) cases, respectively. No tumor showed combined loss of both markers (Figure 1).
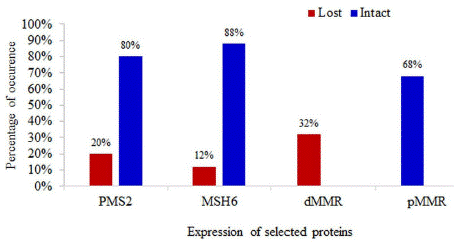
Figure 1: Distribution of the study patients by expression of PMS2 and MSH6 MMR proteins.
Expression of PMS2 and MSH6 MMR proteins in relation to clinicopathological parameters of studied samples are presented in Table 1 while Table 2 shows the association of MMR status with demographic and histomorphologic parameters of the study cases.
Characteristics
Lost PMS2 expression (n=10)
Frequency of Lost PMS2 expression
Intact PMS2 expression (n=40)
Frequency of Intact PMS2 expression
Lost MSH6 expression (n=6)
Frequency of Lost MSH6 expression
Intact MSH6 expression (n=44)
Frequency of Intact MSH6 expression
Age (Years)
<50
8
80%
19
47.5%
5
83.3%
22
50%
=50
2
20%
21
52.5%
1
16.7%
22
50%
Sex
%
Male
7
70%
25
62.5%
6
100.0%
26
59.1%
Female
3
30%
15
37.5%
0
0.0%
18
40.9%
Tumor site
Right colon
4
40%
14
35%
2
33.3%
16
36.4%
Left colon
6
60%
25
62.5%
3
50.0%
28
63.6%
Both
0
0%
1
2.5%
1
16.7%
0
0.0%
Mucinous differentiation
None
4
40%
30
75%
4
66.7%
30
68.2%
1-50%
3
30%
1
2.5%
1
16.7%
3
6.8%
>50%
3
30%
9
22.5%
1
16.7%
11
25.0%
Histologic type
Adenocarcinoma (NOS)
7
70%
31
77.5%
5
83.3%
33
75.0%
Mucinous adenocarcinoma
3
30%
9
22.5%
1
16.7%
11
25.0%
Grading of tumor
I
0
0%
2
5%
1
16.7%
1
2.3%
II
7
70%
29
72.5%
4
66.7%
32
72.7%
III
3
30%
9
22.5%
1
16.7%
11
25.0%
Staging of Tumors (pT)
T1
0
0%
1
2.5%
0
0.0%
1
2.3%
T2
1
10%
10
25%
2
33.3%
9
20.5%
T3
7
70%
27
67.5%
4
66.7%
30
68.2%
T4
2
20%
2
5%
0
0.0%
4
9.1%
Staging of Tumors (pN)
Nx
1
10%
1
2.5%
0
0.0%
2
4.5%
N0
6
60%
18
45%
6
100.0%
18
40.9%
N1
1
10%
10
25%
0
0.0%
11
25.0%
N2
2
20%
11
27.5%
0
0.0%
13
29.5%
Lymphovascular invasion(LVI)
Present
3
30%
26
65%
1
16.7%
28
63.6%
Absent
7
70%
14
35%
5
83.3%
16
36.4%
Crohn like peritumoral reaction
Present
9
90%
9
22.5%
3
50.0%
15
34.1%
Absent
1
10%
31
77.5%
3
50.0%
29
65.9%
Intra-tumoral lymphocytic infiltrate
None
0
0%
7
17.5%
0
0.0%
7
15.9%
Mild to moderate
2
20%
28
70%
2
33.3%
28
63.6%
Marked
8
80%
5
12.5%
4
66.7%
9
20.5%
Table 1: Expression of PMS2 and MSH6 MMR proteins in relation to clinicopathological parameters in Bangladeshi CRC cases.
Characteristics
Total dMMR cases (n=16)
Frequency of Total dMMR cases
Total pMMR cases (n=34)
Frequency of total pMMR cases
P value
Age (Years)
<50
13
81.3%
14
41.2%
0.06
=50
3
18.8%
20
58.8%
Sex
0.117
Male
13
81.3%
19
55.9%
Female
3
18.8%
15
44.1%
Tumor site
Right colon
6
37.5%
12
35.3%
0.322
Left colon
9
56.3%
22
64.7%
Both
1
6.3%
0
0.0%
Mucinous differentiation
None
8
50.0%
26
76.5%
1-50%
4
25.0%
0
0.0%
>50%
4
25.0%
8
23.5%
Histologic type
Adenocarcinoma (NOS)
12
75.0%
26
76.5%
1.0
Mucinous adenocarcinoma
4
25.0%
8
23.5%
Grading of tumor
I
1
6.3%
1
2.9%
0.842
II
11
68.8%
25
73.5%
III
4
25.0%
8
23.5%
Staging of Tumors (pT)
T1
0
0.0%
1
2.9%
0.757
T2
3
18.8%
8
23.5%
T3
11
68.8%
23
67.6%
T4
2
12.5%
2
5.9%
Staging of Tumors (pN)
Nx
1
6.3%
1
2.9%
N0
12
75.0%
12
35.3%
N1
1
6.3%
10
29.4%
N2
2
12.5%
11
32.4%
Lymphovascular invasion(LVI)
Present
4
25.0%
25
73.5%
Absent
12
75.0%
9
26.5%
Crohn like peritumoral reaction
Present
12
75.0%
6
17.6%
0.001
Absent
4
25.0%
28
82.4%
Intra-tumoral lymphocytic infiltrate
None
0
0.0%
7
20.6%
0.001
Mild to moderate
4
25.0%
26
76.5%
Marked
12
75.0%
1
2.9%
Table 2: Association of MMR status with demographic and histomorphologic parameters in Bangladeshi CRC cases.
Age of the study population varied from 19-85 years with a mean of 48.60±14.6 years. A total of 32 male patients and 18 female patients enrolled in the study which made the male and female population 64% and 36%, respectively. However, dMMR status of the tumors did not show any significant association with patients’ age and sex (Table 2).
Only one case had a positive family history of colon cancer among the 50 studied cases which revealed lost expression of MSH6. None of the cases showed a history of extracolonic cancer.
Two histological subtypes of CRC were observed in this study: adenocarcinoma (NOS) and mucinous adenocarcinoma (Figure 2 & Figure 3). Adenocarcinoma (NOS) comprised the majority (75%) of the dMMR cases. However, no significant association was observed between dMMR status the and histological subtype of tumor (Table 2).
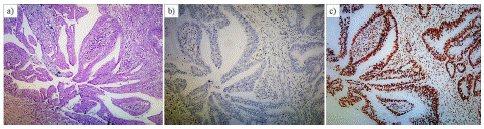
Figure 2: Photomicrograph of section of the resected colon showing (A) Adenocarcinoma (NOS), x100, H&E) (B) Lost expression of PMS2 in tumor cells and intact internal control (lymphocytes and stromal cells), x100, PMS2 immunostain (C) Intact expression of MSH6 in tumor cells and internal control (lymphocytes and stromal cells), x100, MSH6 immunostain.
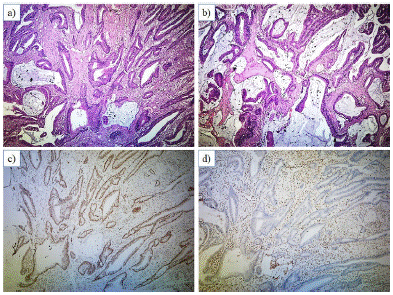
Figure 3: Photomicrograph of section of the resected colon showing (A, B) Mucinous adenocarcinoma; x40, H&E (C) Intact expression of PMS2 in tumor cells and internal control (lymphocytes and stromal cells), x40, PMS2 immunostain) (D) Lost expression of MSH6 in tumor cells and intact internal control (lymphocytes and stromal cells), x40, MSH6 immunostain.
Among the dMMR cases, 25% of tumors showed mucinous histology with extracellular mucin involving more than 50% of the tumor area. Another 25% of cases had mucinous histology having extracellular mucin in 1-50% of the tumor area. No extracellular mucin production was seen in the rest 50% of cases. However, no significant association between dMMR status and mucinous histology was observed (Table 2).
Considering the depth of invasion for staging (pT), dMMR tumors were observed to be at stage T3 in most (68.8%) of the cases. But, no significant association of dMMR tumors with pT staging was observed (Table 2).
A statistically significant association (p=0.042) of dMMR cases with a lower number of lymph node metastasis was observed. The prevalence of dMMR cases found at the N0 stage was 75% in the study. The remaining 6.3%, 6.3% and 12.4% cases were recorded at stages Nx, N1, and N2, respectively (Table 2). In majority (75%) of the dMMR tumors, Lymphovascular Invasion (LVI) was recorded to be absent having a significant association of dMMR CRC with a lower risk of LVI (P=0.002).
Among the dMMR tumors, Crohn-like peritumoral reaction was present in 75% of cases and absent in 25% of cases. In 28 (82.4%) cases of pMMR this feature was absent. A significant association between MMR status of CRC with Crohn like peritumoral reaction was recorded (P=0.001). Marked intratumoral infiltrate was present in 75% of dMMR cases while mild to moderate infiltrate was observed in four 25% of these cases. No dMMR case was observed without intratumoral lymphocytic infiltrate. A significant association of dMMR cases with marked intra-tumoral lymphocytic infiltrate was observed (P=0.001). However, no significant association between MMR status and tumor location or tumor grade was observed.
Discussion
The present study was carried out to unveil the immunohistochemical expression of PMS2 and MSH6 DNA mismatch repair proteins in CRC patients to predict the dMMR CRC cases due to the loss of these proteins. This study also investigated the association of the expression of these selected proteins with several clinicopathological parameters (age, sex, tumor location, microscopic features, histological subtype, grade, stage and features of host immune response, etc.).
A total of 32% of CRC cases were found to be dMMR due to the loss of any one of the selected proteins. Loss of expression of PMS2 and MSH6 were observed in 20% and 12% of cases, respectively. The frequency of dMMR CRC cases was found to be variable in different studies on different population, like- USA (13%) [22], China (6.7%) [23], Australia (18%) [24], India (29%) [25], Pakistan (34%) [18] etc. The similarity of the current study findings with Indian and Pakistani populations may be due to similar ethnicity, food habit and environment. However, a study conducted by Rahman, 2014 on 39 Bangladeshi CRC patients recorded the frequency of MSI tumors as 22.5% by PCR [26]. The difference in MSI detection techniques and sample size might have influenced the result. In this study, loss of expression of PMS2 was a more common observation than that of MSH6. This finding matches the result of several studies conducted else where [13,18,27-29]. The major obligatory partner for PMS2 is MLH1, the most frequently inactivated gene in dMMR colon cancer. Therefore, the loss of MLH1 leaded to the loss of PMS2 [13-16].
As MLH1 and MSH2 immunostaining were not performed in this study, segregation of the cases with concurrent loss of MLH1/PMS2 from the cases with isolated loss of expression of PMS2 and MSH6 was not possible. A study in the Australian population reported no case with isolated loss of MLH1 or MSH2 and concluded that a panel of two antibodies could be successfully used instead of four antibodies for the initial screening of CRC patients for Lynch syndrome [14]. These findings were also supported by other studies conducted elsewhere [15,16]. Although it is expected that loss of MLH1 and MSH2 will cause degradation of PMS2 and MSH6, respectively, some studies suggest that isolated loss of MLH1 [18] and MSH6 [30] can also occur. This type of expression, if present, might have been missed in the current study, raising the possibility of a higher frequency of dMMR cases. Specific microscopic features, like mucinous histology, signet ring and medullary morphology were carefully searched and only the cases with mucinous histology were noticed. The dMMR status of CRC was significantly associated with mucinous histology. Studies conducted elsewhere also had similar observations indicating that dMMR tumors tend to possess mucinous histology [23-31].
The dMMR CRCs are usually associated with higher histologic grade and early-stage of tumors [32]. In this study, majority of both dMMR and pMMR cases were in stage 3 (T3) based on the pT staging of tumor. No significant association was observed between the expression of the selected MMR proteins and pT staging of the tumor. Due to poverty, ignorance and lack of routine screening programs for early cancer detection, patients’ treatment becomes delayed which causes the progression of the disease to an advanced stage. When staging on the basis of nodal metastasis (pN) was assessed, 75% of the dMMR cases were found to be at the N0 stage. dMMR status of the tumors was found to be significantly associated with lower events of nodal metastasis. In the current study, we observed a significant association of dMMR CRC cases with lower occurrences of LVI (p=0.002). This finding is in good agreement with the studies conducted on Pakistani and Indian populations [18-25]. It seems that the dMMR CRCs are less likely to have LVI. In addition, there might be a racial influence that results in lower occurrences of LVI in the Indo-Pak subcontinental population.
The dMMR colorectal cancers often induce a host immune response resulting in the migration of activated T cells into neoplastic epithelium [33]. According to Greenson et al., 2009 intratumoral lymphocytic infiltrates can accurately classify tumors as MSI-H with approximately 85% probability [34]. In this study, a significant association of the dMMR status of CRC with marked intratumoral lymphocytic infiltrate was observed (p = 0.001). Present study findings also indicated that the presence of marked intratumoral lymphocytic infiltrate in histologic sections might predict MSI-H tumors. Greenson, et al. 2009 concluded that the presence of peritumoral Crohn-like lymphocytic response as a sensitive marker for MSI-H tumors [34]. In our study, a significant association between dMMR CRC and the presence of Crohn-like peritumoral lymphocytic response was recorded. This might be an indication of a strong host immune response to dMMR CRC cases.
Among the total 50 cases, only one case had a family history of colon cancer which showed lost MSH6 expression indicating tumors with MSH2/MSH6 were more prone to have inherited cancer susceptibility. None of the cases presented with a history of extracolonic malignancy. Other established features of MSI like female gender, right-sided location, etc. were not observed in this study. Similarly, the histologic subtype and grade of the tumor didn’t show any significant association with dMMR status.
Conclusion
Microsatellite Instability (MSI) is a key biomarker in Colorectal Cancer (CRC) having crucial diagnostic, prognostic and predictive implications. Testing for mismatch repair deficiency (dMMR)/MSI is recommended during screening for Lynch syndrome characterized by germline mutations in the MMR genes and associated with an increased risk for several types of cancer. The frequency (32%) of dMMR CRCs was comparatively higher in this study population than in other races. As MSI has emerged as a predictor of sensitivity to immunotherapy-based treatments, routine screening of CRC cases for detection of MMR status of tumors by an immunohistochemical method using at least two, preferably all four antibodies is strongly proposed.
Author Statements
Acknowledgments
The authors gratefully acknowledge the logistics and grant support to conduct the research activities from Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Availability of Data and Materials
Raw data, supplemental data, and materials are available on request.
Ethical Consideration
Written consent from individual patient/representatives of patients was obtained for using the samples for research purposes. No personal data/information of patients was shared in public.
Author’s Contribution
SSUM planned, designed and performed the study. She also wrote the manuscript (MS). FB & MMR helped in planning & designing of the study and developing the research question. TI, USS, UTN, SA, helped in grossing of specimens, reviewing slides and literature searches. KBMS helped in study design, data screening and performed data analyses, interpretation and MS writing. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest in publishing the article.
References
- IARC. GLOBOCAN 2020: New Global Cancer Data. 2020. Available from: https://www.uicc.org/news/globocan-2020-new-global-cancer-data.
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017; 66: 683-691.
- Wong MC, Ding H, Wang J, Chan PS, Huang J. Prevalence and risk factors of colorectal cancer in Asia. Intestinal Research. 2019; 17: 317-329.
- Al-Sohaily S, Biankin A, Leong R, Corish MK, Warusavitarne J. Molecular pathways in colorectal cancer. Journal of Gastroenterology and Hepatology. 2012; 27: 1423-31.
- Müller A, Edmonston TB, Dietmaier W, Büttner R, Fishel R, et al. MSI-testing in hereditary non-polyposis colorectal carcinoma (HNPCC). Disease Markers. 2004; 20: 225-36.
- Kunkel TA. Evolving views of DNA replication (in)fidelity. Cold Spring Harbor Symposia on Quantitative Biology. 2009; 74: 91–101.
- Fishel R, Lescoe MK, Rao M, Copeland G, Jenkins A, et al. The Human Mutator Gene Homolog MSH2 and Its Association with Hereditary Nonpolyposis Colon Cancer. Cell. 1993; 75: 1027-1038.
- Turner JR. The Gastrointestinal Tract. In: Robbins and Cotran Pathologic Basis of Disease. 9th ed.: Elsevier; 2014; 749-819.
- Lothe RA, Peltomäki P, Meling GI, Aaltonen LA, Nyström-Lahti M, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. The Journal of Cancer Research. 1993; 53: 5849–52.
- Sengupta SB, Yiu CY, Boulos PB, De Silva M, Sams VR, et al. Genetic instability in patients with metachronous colorectal cancers. British Journal of Surgery. 1997; 84: 996–1000.
- Carethers JM, Chauhan DP, Fink D, Nebel S, Bresalier RS, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999; 117: 123–31.
- Shia J, Holck S, Depetris G, Greenson JK, Klimstra DS. Lynch syndrome-associated neoplasms: A discussion on histopathology and immunohistochemistry. Familial Cancer. 2013; 12: 241-60.
- Shia J, Tang LH, Vakiani E, Guillem JG, Stadler ZK, et al. Immunohistochemistry as first-line screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: a 2-antibody panel may be as predictive as a 4-antibody panel. American Journal of Surgical Pathology. 2009; 33: 1639-45.
- Hall G, Clarkson A, Shi A, Langford E, Leung H, et al. Immunohistochemistry for PMS2 and MSH6 alone can replace a four antibody panel for mismatch repair deficiency screening in colorectal adenocarcinoma. Pathology. 2010; 42: 409–413.
- Mojtahed A, Schrijver I, Ford JM, Longacre TA, Pai RK. A two-antibody mismatch repair protein immunohistochemistry screening approach for colorectal carcinomas, skin sebaceous tumors, and gynecologic tract carcinomas. Modern Pathology. 2011; 24: 1004-14.
- O’Regan T, Chau K, Tatton M, Smith T, Parry S, Bissett I. Immunochemistry screening for Lynch syndrome in colorectal adenocarcinoma using an initial two antibody panel can replace a four antibody panel. The New Zealand Medical Journal. 2013; 126: 70–7.
- Shiraj-Um-Mahmuda S, Begum F, Rahman MM, Rahman P, Islam T, Shabnam US, et al. Demographic and Clinicopathological Evaluation of Colorectal Adenocarcinoma in Bangladesh at a Tertiary Level Hospital. Cancer Studies and Therapeutics Journal. 2023; 8: 1-8.
- Hashmi AA, Ali R, Hussain ZF, Faridi N, Khan EY, et al. Mismatch repair deficiency screening in colorectal carcinoma by a four-antibody immunohistochemical panel in Pakistani population and its correlation with histopathological parameters. World Journal of Surgical Oncology. 2017; 15: 116.
- Ogino S, Kawasaki T, Nosho K, Kirkner GJ, Fuchs CS. LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. International Journal of Cancer. 2008; 122: 2767-2773.
- Graham M, Appelman. Crohn’s-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Modern Pathplogy. 1990; 3: 332-335.
- Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, et al. Lymphocytic Reaction to Colorectal Cancer Is Associated with Longer Survival, Independent of Lymph Node Count, Microsatellite Instability, and CpG Island Methylator Phenotype. Clinical Cancer Research. 2009; 15: 6412-6420.
- Ricker CN, Hanna DL, Peng C, Nguyen NT, Stern MC, et al. DNA mismatch repair deficiency and hereditary syndromes in Latino patients with colorectal cancer. Cancer. 2017; 123: 3732–3743.
- Hu XR, Xu C, Kang Y, Wang T, Zhang Y, et al. Correlation between mismatch-repair protein expression and clinicopathologic features in 658 colorectal cancers. Chinese Journal of Pathology. 2018; 47: 827-833.
- Loh Z, Williams S, Salmon L, Dow E, John T. Impact of universal immunohistochemistry on Lynch syndrome diagnosis in an Australian colorectal cancer cohort. Journal of Internal Medicine. 2019; 49: 1278-1284.
- Rai PR, Shetty N, Rai PR, Shet D, Shetty A. A study on the frequency and clinicopathological correlates of mismatch repair-deficient colorectal cancer. Journal of Cancer Research & Therapeutics. 2020; 16: S183-S188.
- Rahman MM. Expression pattern of the proliferative marker Ki-67 and status of microsatellite instability in different histomorphological patterns of colorectal carcinoma MD thesis. 2014.
- Molaei M, Mansoori K, Ghiasi S, Zali MR. Colorectal cancer in Iran: immunohistochemical profiles of four mismatch repair proteins. International Journal of Colorectal Disease. 2010; 25: 63-69.
- Reverón D, López C, Gutiérrez S, Malafa M, Coppola D. Frequency of Mismatch Repair Protein Deficiency in a Puerto Rican Population with Colonic Adenoma and Adenocarcinoma. Cancer Genomics and Proteomics. 2018; 15: 265-271.
- Cheah PL, Li J, Looi LM, Koh CC, Lau TP, et al. Screening for microsatellite instability in colorectal carcinoma: Practical utility of immunohistochemistry and PCR with fragment analysis in a diagnostic histopathology setting. Malaysian Journal of Pathology. 2019; 41: 91-100.
- Pearlman R, Markow M, Knight D, Chen W, Arnold CA, et al. Two-stain immunohistochemical screening for Lynch syndrome in colorectal cancer may fail to detect mismatch repair deficiency. Modern Pathology. 2018; 31: 1891-1900.
- Kaur G, Masoud A, Raihan N, Radzi M, Khamizar W, et al. Mismatch repair genes expression defects & association with clinicopathological characteristics in colorectal carcinoma. The Indian Journal of Medical Research. 2011; 134: 186-192.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. Journal of Clinical Oncology. 2005; 23: 609-18.
- Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. American Journal of Surgical Pathology. 1999; 154: 1805-1813.
- Greenson JK, Huang SC, Herron C, Moreno V, Gruber SB. Pathologic predictors of microsatellite instability in colorectal cancer. American Journal of Surgical Pathology. 2009; 33: 126-133.