
Research Article
Ann Hematol Onco. 2023; 10(3): 1428.
Effectiveness of Random Donor Platelet (RDP) Transfusion Therapy in Acute Leukaemia Patients: A Retrospective Analysis
Neelesh Jain, MD¹*; Dibyendu De, DM²; Ruchi Aujla, MD³
1Department of Transfusion Medicine & Blood Centre Sector 36, Naya Raipur, Chhattisgarh, India
2Department of Haematology, Balco Medical Centre, (A unit of Vedanta Medical Research Foundation), India
3Department of Paediatrics, Balco Medical Centre, (A unit of Vedanta Medical Research Foundation), India
*Corresponding author: Neelesh Jain Department of Transfusion Medicine & Blood Centre Sector 36, Naya Raipur, Chhattisgarh, 493661, India. Email: drneeleshjain@gmail.com
Received: May 03, 2023 Accepted: June 01, 2023 Published: June 08, 2023
Abstract
Introduction: In India, lympho-hematopoietic malignancies constitute 9.5% of all cancers in men and 5.5% in women. The incidence of ALL and AML are 35% and 15% of all hematological malignancies respectively. Acute leukemia patients require multiple transfusions for a prolonged period of time. Patients undergoing induction and/or consolidation chemotherapy for leukemia often require platelet transfusion at least every 3 days. Normal platelet survival is approximately 5-7 days. Long - term platelet supportive care may be complicated by the development of a state of refractoriness, resulting in inadequate recovery of platelets. Moreover, with the progress of medical technology, platelet transfusion therapy is gradually getting better in the treatment of leukemic patients.
Aim: To study random donor platelet transfusion efficacy in acute leukaemia patients.
Objective: To study platelet transfusion threshold.
To study adequate dosing of platelet concentrate transfused.
Material and Methods: This is a 1-year observational study undertaken in the department of Transfusion Medicine and Hematology of our Centre at Raipur, Chhattisgarh, India. The study population included acute leukemia patients who had been transfused with platelet concentrates (i.e. Random Donor Platelet or RDP) and whose repeat platelet count was done 24 hours post transfusion. Threshold for platelet transfusion was kept at twenty thousand platelet count per microliter or active bleeding irrespective of platelet count. The study group included patients of both sexes and of all age groups.
Results: A total of 52 acute leukaemia patients (demographic details & clinical characteristics are given in the table 1&2) received 785 Platelet concentrate units (RDP) at 211 occasions with a median of 10.5 units RDP given to each patient in the median of 3 episodes of transfusion. The mean value of 24hrs Post Transfusion Platelet Increment (PPI) was 9655/μl (Figure 1). Seventeen(32.6%) patients showed 24 hrs PPI value <4500/μl. Overall 31(59.6%) patients developed blood and/or urine culture positive sepsis during the course of treatment, of which nine (52.9%) patients belongs to the category whose PPI value was <4500/μl. At 39 occasions patient received RDP units prepared within 24 hours of whole blood collection. All patients received non ABO identical group but compatible platelet transfusion at least at one occasion. Males (59.6%) showed high post transfusion platelet increment then females (40.4%) (i.e. 10420 v/s 8890). Dose adequacy was noticed in 66.82% of events of transfusion with 67.40% of response adequacy (Figure 2). Seven patients experienced Febrile non Haemolytic Transfusion (FNHTR) reactions. Refractoriness could not be identified as 1 hour post transfusion platelet count was not done. AML patients showed better post transfusion platelet response then ALL. Mean platelet count per RDP unit was 6.45X1010.
Age (years)
ALL
AML
Male
Female
Male
Female
=15 years
9
7
4
3
16-60 years
5
2
9
7
>60 years
2
1
2
1
Table 1: Age and Gender distribution.
Clinical features
ALL (n=26)
AML (n=26)
n
%
n
%
Fever
11
42
20
77
Hepatomegaly
16
62
13
50
Splenomegaly
19
73
15
58
Lymphadenopathy
7
27
1
3.8
Bleeding
2
7.6
5
19
Table 2: Clinical characteristics.
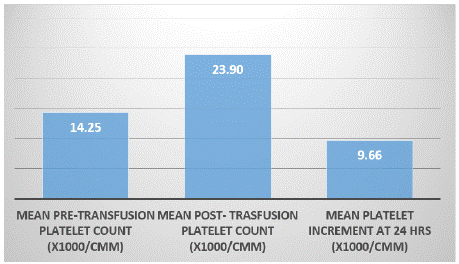
Figure 1: Mean platelet increment.
Conclusion: In the current study, it was found that fever and infection were the main determinants of transfusion efficacy. Two third of our study population received adequate platelet transfusion with 67.4% showed adequate platelet recovery after 24hours of transfusion. To further assess the influence of patient and disease variables on platelet efficacy in hemato-oncology patients, more studies with a larger sample size need to be conducted.
Introduction
In India, lympho-haematopoietic malignancies constitute 9.5% of all cancers in men and 5.5% in women [1]. The incidence of ALL and AML are 35% and 15% of all hematological malignancies respectively [2]. Primary and immature leukaemia cells accumulate in the body following malignant proliferation, interfering with normal haematological function. Those with clinical leukaemia frequently have anaemia, haemorrhage, infection, and extramedullary infiltration [3-5]. When major bleeding is not promptly treated, it can result in a significant drop in platelets and patient death. The primary cause of haemorrhage in acute leukaemia patients is the destruction of platelets induced by bone marrow haematological malfunction and chemotherapy drug toxicity [6]. Leukemia remission rates have improved dramatically, however there are still issues with increasing comorbidities, a high recurrence rate, patients with weakened immune systems, and thrombocytopenia, which has not yet been fully resolved [7-8]. In order to prevent death caused by excessive blood loss in patients with leukemia, platelet decline can be prevented and patients can be treated with platelet transfusion repeatedly. Acute leukemia patients require multiple transfusions for a prolonged period of time. Patients undergoing induction and/or consolidation chemotherapy for leukemia often require platelet transfusion at least every 3 days. Normal platelet survival is approximately 5-7 days. Long-term platelet supportive care may be complicated by the development of a state of refractoriness, resulting in inadequate recovery of platelets. Moreover, with the progress of medical technology, platelet transfusion therapy is gradually getting mature in the treatment of leukemia patients. The incidence of ineffective platelet transfusion continues to rise including platelet refractoriness, and even leads to death in severe cases [9].
Materials and Methods
Inclusion and exclusion criteria
Patients who were diagnosed with acute leukemia by pathological features and met the Diagnostic Criteria for Leukemia and received random donor platelet transfusion during the course of their treatment are included in the study [10,11]. Patients with incomplete clinical data and patients with diagnosis other than acute leukemia are excluded from the study.
RDP Preparation
The platelet concentrate was prepared by Platelet Rich Plasma (PRP) method and were stored at 20-24*C (shelf life 5 days) under constant gentle agitation. The platelets were neither leukoreduced nor irradiated. No apheresis platelets were used in the study population of patients. The bleeding event were categorized based on WHO grading of symptoms as no bleeding, non-clinically significant bleeding and clinically significant bleeding. The transfusion was given depending upon the clinical sign of bleeding and the platelet count [12]. The pre and post transfusion platelet count were estimated at least 24hours after the transfusion using hematology analyzer. All patient received ABO non identical but compatible platelet transfusion at least once during the course of treatment.
Patients & product characteristics evaluated
Baseline patient factors considered were age, gender, history of pregnancy or previous transfusion, height, weight, and previous splenectomy. Patient characteristics evaluated for each transfusion were palpable spleen, presence of bleeding, fever, infection, transfusion reaction, or DIC. The characteristics of the transfused platelets that were analysed were; product platelet count; ABO compatibility; preparation method of the platelet product, whether the platelet product was γ-irradiated, volume-reduced, or fresh (transfused within 48 hours of collection).
Platelet transfusions method & dosing
Transfusion was conducted strictly in accordance with the hospital transfusion policy. Blood grouping and antibody screening of patients was checked and recorded, and blood matching was performed just prior to transfusion. During transfusion, patients tolerance was observed to timely adjust the speed of transfusion. The transfusion was completed within 30-40 minutes on average. Most patients received prophylactic platelet transfusions for platelet counts of less than or equal to 20×10/L, or at higher levels for particular clinical indications; for example, active bleeding. Dose of platelet transfusion-A standard dose of 0.5x1011 platelets per 10kg body weight of patient or standard amount of adult transfusion was one curative dose of platelet suspension (6units, 250-300ml) [13]. The Post-Transfusion Platelet Increment (PPI) is the difference in platelet count between pre- and post-transfusion. In this study PPI at 24 hours was calculated to understand the efficacy of platelet transfusion.
Results
A total of 52 acute leukaemia patients (demographic details & clinical characteristics are given in the table 1&2) received 785 Platelet concentrate units (RDP) at 211 occasions with a median of 10.5 units RDP given to each patient in the median of 3 episodes of transfusion. The mean value of 24hrs Post transfusion Platelet Increment (PPI) was 9655/μl (Figure 1). Seventeen (32.6%) patients showed 24hrs PPI value<4500/μl. Overall 31(59.6%) patients developed blood and/or urine culture positive sepsis during the course of treatment, of which nine (52.9%) patients belongs to the category whose PPI value was <4500/μl. At 39 occasions patient received RDP units prepared within 24 hours of whole blood collection. All patients received non ABO identical group but compatible platelet transfusion at least at one occasion. Males (59.6%) showed high post transfusion platelet increment then females (40.4%) (i.e. 10420 v/s 8890). Dose adequacy was noticed in 66.82% of events of transfusion with 67.40% of response adequacy (Figure 2). Seven patients experienced Febrile Non Haemolytic Transfusion Reactions (FNHTR). Refractoriness could not be identified as 1 hour post transfusion platelet count was not done. AML patients showed better post transfusion platelet response then ALL. Mean platelet count per RDP unit was 6.45X1010.
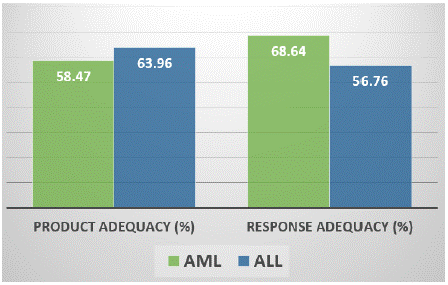
Figure 2: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
Discussion
Hemato-oncology services require many transfusions for a prolonged period. Normal platelet survival is approximately nine days. Hanson SR et al., suggested that patients undergoing induction chemotherapy for leukemia often require platelet transfusion at least every three days [14]. Pattern E et al., published that acute leukemic patients receive on average 80-110 units of platelets [15]. In the present study, 785 random donor platelet transfusions were given to 52 acute leukemic patients with an average of 15 units per patient. In contrast to a therapeutic method, where platelet transfusions are given after a particular level of haemorrhage has occurred, a preventative platelet transfusion approach can stop bleeding. The majority of the recommendations for preventive platelet transfusion are based on clinical experience. There are two conflicting points of view in cancer patients. One group advises transfusions whenever the platelet count drops below 20000/μL, while the other group feels that transfusions should only be given when there is actual bleeding. Bayer wL et al., found that patients with platelet counts less than 6000/μL received prophylactic transfusion, where as those with counts greater than 20000/μL were transfused only for major bleeding. They concluded that prophylactic level of 5000/μL was safe in the absence of fever or bleeding [16]. Gmur et al., Heckman et al, Rebulla et al., have compared the bleeding risk and platelet transfusion needs of groups of thrombocytopenic patients’ who received platelets either at the 10000/μL or 20000/μL threshold [17,18]. They found that there is no difference in hemorrhagic morbidity and mortality rates when the lower platelet transfusion trigger values are used. One major reason for variable practice is based on the need to modify threshold numbers when thrombocytopenia is combined with other complications that increase the risk of bleeding. In the present study the transfusion trigger of platelet count <20,000/μL was followed. There are multiple factors including immunological, non-immune as well as platelet related characteristics which are responsible for Post transfusion Platelet Increment (PPI) as shown in Table 3. Janice P Dutcher et al., studied in 114 patients with acute lymphoblastic leukemia who received multiple course of chemotherapy and several platelet transfusions and found that 92% of the patients never become alloimmunized and responded to random donor platelets. There was no difference in age or sex between groups and prognostic factors predicting alloimmunization [19]. Dutcher in his previous studies also found that there is no dose response relationship between the development of alloimmunization and the number of units of platelets given during induction. In our study refractoriness could not be identified as 1 hour post transfusion platelet count was not done. The mean value of 24 hrs Post Transfusion Platelet Increment (PPI) was 9655/μl. Seventeen (32.6%) patients showed 24 hrs PPI value <4500/μl [20]. McCullough J documented in 2000 that the use of platelet transfusion is associated with increased risk of viral and bacterial infection and alloimmunization. In the present study of fifty-two acute leukemic patients, all were negative for transfusion transmissible infections. He also found that transfusion reaction occurs after 5% to 30% of platelet transfusion and the most common adverse reaction is febrile non-hemolytic transfusion reaction, which is caused by the patient’s leukocyte antibodies reacting with leucocytes in the transfused components. In the present study out of 52 patients who received platelet transfusion, seven of them experienced febrile non-hemolytic transfusion reaction [21].
Nonimmune causes
Immune causes
Platelet conc. characteristics
Sepsis
HLA alloimmunization
Donor-recipient ABO compatibility
Fever
HPA alloimmunization
Duration of platelet storage
DIC
ABO incompatibility
Platelet dose
Splenomegaly
Autoantibodies
Platelet source
Drugs (e.g. vancomycin, amphotericin B)
Leukocyte reduction
Table 3: Factors affecting post transfusion platelet counts.
Conclusion
Platelet transfusions have considerably improved the clinical management of acute leukaemia patients, which has led to a dramatic decline in the mortality rate from bleeding complications and good clinical outcomes. Platelet transfusions can have both immune-mediated and non-immune-mediated side effects, despite the fact that they can save lives. In the current study, it was found that fever and infection were the main determinants of transfusion efficacy. Two third of our study population received adequate platelet transfusion with 67.4% showed adequate platelet recovery after 24 hours of transfusion. To further assess the influence of patient and disease variables on platelet efficacy in hemato-oncology patients, more studies with a larger sample size need to be conducted.
References
- National cancer Registry programme, consolidated report of population based cancer registries 1990-96. Incidence and distribution of cancer Indian council of Medical Research. New Delhi, Aug 2001.
- Ahirwar R, Nigam RK, Parmar D. A study of leukemias Profile in central India. Trop J Path Micro. 2018; 4: 182-187.
- Maertens J, Marchetti O, Herbrecht R, Cornely OA, Fluckiger U, et al. Third European Conference on Infections in Leukemia: European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: Summary of the ECIL 3-2009 update. Bone Marrow Transplant. 2011; 46: 709-718.
- Takami A, Yano S, Yokoyama H, Kuwatsuka Y, Yamaguchi T, et al. Donor lymphocyte infusion for the treatment of relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: A retrospective analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2014; 20: 1785-1790.
- Roe JS, Mercan F, Rivera K, Pappin DJ, Vakoc CR: BET bromodomain inhibition suppresses the function of hematopoietic transcription factors in acute myeloid leukemia. Mol Cell. 2015; 58: 1028-1039.
- Houwerzijl EJ, Blom NR, van der Want JJ, Louwes H, Esselink MT, et al. Increased peripheral platelet destruction and caspase-3-independent programmed cell death of bone marrow megakaryocytes in myelodysplastic patients. Blood. 2005; 105: 3472-3479.
- Frinc I, Dima D, Chitic M, Berce C, Berindan-Neagoe I, et al. Transthoracic ultrasonography for the follow-up of a chronic lymphocytic leukemia patient with chemotherapy-induced immunosuppression prior to allogeneic stem cell transplantation. A case report. Med Ultrason. 2017; 19: 330-332.
- Just Vinholt P, Hojrup Knudsen G, Sperling S, Frederiksen H; Nielsen C. Platelet function tests predict bleeding in patients with acute myeloid leukemia and thrombocytopenia. Am J Hematol. 2019; 94: 891-901.
- Apelseth TO, Tor Hervig MD, Oystein Bruserud MD. Platelet transfusion in acute leukemia patients with severe chemotherapy-induced thrombocytopenia: The possible importance of hemoglobin levels and red blood cell transfusions for evaluation of clinical effects of transfusion. Transfusion. 2010; 50: 2505-2506.
- Mason J, Griffiths M. Molecular diagnosis of leukemia. Expert Rev Mol Diagn. 2012; 12: 511-526.
- Gmur J, Burger J, Schanz U, Fehr J, Schaffner A. Safety of stringent prophylactic platelet transfusion policy for patients with acute leukaemia. Lancet. 1991; 338: 1223-1226.
- Subash S, Umesh D. Effectiveness of platelet transfusion in acute leukemic pediatric patients: a prospective study. Int J Contemp Pediatr. 2018; 5: 824-828.
- Heim D, Passweg J, Gregor M, Buser A, Theocharides A, et al. Patient and product factors affecting platelet transfusion results. Transfusion. 2008; 48: 681-687.
- Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood. 1985; 66: 1105-1109.
- E. Patten. Controversies in transfusion medicine. Prophylactic platelet transfusion revisited after 25 years. Transfusion. 1992; 32: 381-385.
- Bayer WL, Bodensteiner DC, Tilzer LL, Adams ME. Use of platelets and other transfusion products in patients with malignancy. Semin Thromb Hemost. 1992; 18: 380-391.
- Heckman KD, Weiner GJ, Davis CS, Strauss RG, Jones MP, et al. Randomized study of prophylactic platelet transfusion threshold during induction therapy for adult acute leukemia: 10,000/microL versus 20,000/microL. J Clin Oncol. 1997; 15: 1143-1149.
- Rebulla P, Finazzi G, Marangoni F, Avvisati G, Gugliotta L, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. N Engl J Med. 1997; 337: 1870-1875.
- Janice P Dutcher, Charles A Schiffer, Joseph Aisner, Peter H Wiernick. Long term follow-ups of patients with Leukemia Receiving Platelet Transfusions: Identification of a Large Group of Patients who denote become allo immunized. Blood. 1981; 58: 1007-1010.
- Janice P Dutcher, Charles A Schiffer, Joseph Aisner, Peter H Wiernick. Long term follow-ups of patients with Leukemia Receiving Platelet Transfusions: Identification of a Large Group of Patients who denote become allo immunized. Blood. 1981; 58: 1007-1010.
- Jeffrey Mc Cullough. Current issues with platelet transfusion in patients with cancer. Semin Hematol. 2000; 37: 3-10.