
Case Report
Ann Hematol Onco. 2023; 10(6): 1443.
Recurrent Massive Gastrointestinal Bleeding as a first presentation of AL Amyloidosis in a 76-Year-Old Patient
Eilam Rabina1; Sagee Tal2; Yaron Rudnicki3; Assaf Rahmani3; Timna Naftali4,6; Martin H Ellis5,6; Osnat Jarchowsky Dolberg1,5,6
1Internal medicine A ward, Meir Medical Center, Kfar Saba Israel
2Pathology Institute, Meir Medical Center, Kfar Saba Israel
3General surgery B ward, Meir Medical Center, Kfar Saba Israel
4Gastroenterology and Hepatology Institute, Meir Medical Center, Kfar Saba Israel
5Hematology Institute, Meir Medical Center, Kfar Saba Israel
6Tel Aviv University, Israel
*Corresponding author: Osnat Jarchowsky Dolberg Internal medicine A ward, Meir Medical Center, 59 Tchernichovsky St, Kfar Saba 44281, Israel Tel Aviv University, Israel. Email: Osnat.jarchowsky@clalit.org.il
Received: November 28, 2023 Accepted: December 21, 2023 Published: December 28, 2023
Abstract
Background: Light chain or AL amyloidosis is a systemic disease that occasionally involves the gastrointestinal tract, but very rarely presents as gastrointestinal bleeding without evidence of systemic involvement. In such cases recognition of amyloidosis as the cause of bleeding is essential in order to rapidly initiate effective treatment to stop the bleeding.
Case presentation: We describe a 76-year-old patient who presented with recurrent massive gastrointestinal bleeding as the initial manifestation of AL amyloidosis. One episode necessitated emergency right colectomy, and histopathological analysis of the resected bowel revealed diffuse amyloid infiltration. This finding prompted further investigations, which demonstrated elevated plasma lambda light chain levels, and 10% monoclonal plasma cells in the bone marrow. Treatment with bortezomib, dexamethasone, and daratumumab was initiated and no further gastrointestinal bleeding occurred.
Conclusion: The diagnosis of amyloidosis involving the gastrointestinal tract should be considered in adult patients with unexplained recurrent gastrointestinal bleeding.
Background
Gastrointestinal Bleeding (GIB) has various etiologies, the commonest being peptic ulcer disease and diverticulosis [1,2].
Amyloid Light chain (AL) amyloidosis, characterized by the deposition of proteins derived from Immunoglobulin (Ig) Light Chains (LCs) in a variety of solid organs, is a rare condition [3]. Involvement of the Gastrointestinal Tract (GIT) in this disorder is not uncommon [4,5]; yet few cases of GIB as the initial presentation of amyloidosis have been reported [6,7], and none in which recurrent massive GIB is the sole clinical manifestation.
In this report, we present a rare case of recurrent massive GIB that initially eluded explanation. Subsequently, the patient was diagnosed with AL amyloidosis, with diffuse GIT involvement which was the source of GIB. Appropriate treatment for amyloidosis was initiated with a salutary effect on the bleeding.
Case Presentation
A 76-year-old patient with a history of Atrial Fibrillation (AF) managed with rivaroxaban presented to the Emergency Department (ED) with massive hematochezia. This resulted in hemorrhagic shock with a hemoglobin (Hb) level of 4.5g/dL (normal range: 12–16g/dL). He was treated with systemic tranexamic acid, multiple blood transfusions were administered and rivaroxaban was discontinued. Emergency upper endoscopy was normal and colonoscopy revealed two, 0.8 cm long, polypoid protrusions, a round solid 4 cm-diameter submucosal mass with a bleeding marker in the ascending colon, and a 2 cm long clot attached to the mucosa near the hepatic flexure (Figure 1). Biopsies were taken from the polyps and from the submucosal mass showed mild inflammation only. One month later, the patient again presented to the ED with hemorrhagic shock due to GIB. Computer tomogram angiography identified active colonic bleeding near the hepatic flexure, thickening of terminal ileum and cecum wall, and enlarged abdominal lymph nodes (Figure 2). Emergency right hemicolectomy and diversion ileostomy were performed. Histology of the resected colon and ileum of diffuse submucosal infiltration of amorphous material that was positive for amyloid with Congo red staining under polarized light. Amyloid deposits were also found in many of the 42 peri-colonic lymph nodes that were resected.
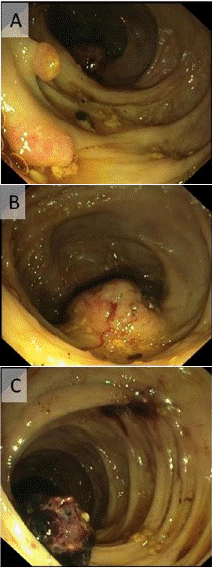
Figure 1: Endoscopic view of the right colon: 0.8 cm long polypoid protrusions (A), solid localized elevated submucosal tumor-like lesion, 4 cm diameter (B), a 2 cm long clot connected to colonic mucosa (C).

Figure 2: Coronal (A) and Axial (B) CTA views shows blush of contrast fluid into the hepatic flexure of the colon, And (C) axial view of enlarged lymph nodes in the right meso-colon.

Figure 3: Amorphous eosinophilic material in the submucosa and in blood vessel walls (A, X100, Hematoxylin and Eosin), Congo red stain of the colon, suggestive of amyloid (B, X100).
Based on these results, further investigation for systemic amyloidosis revealed serum lambda LCs levels of 753 mg/L (normal range: 5.7-26 mg/L). Bone marrow biopsy was performed, demonstrating 10% monoclonal plasma cells. 11% of these cells had
Following the diagnosis of AL amyloidosis with GIT involvement, treatment with bortezomib and dexamethasone was initiated. However, after the second course, lambda LC levels remained unchanged, and the patient had another episode of GIB that required blood transfusion. Therefore, the treatment was escalated with the addition of daratumumab to the current treatment. Following this the patient's hemoglobin stabilized, and GIB ceased. This was accompanied by a decrease in lambda LCs to normal levels of 16 mg/L.
Discussion
Amyloidosis encompasses a wide range of disorders characterized by the deposition of misfolded extracellular proteins in various tissues [8]. It includes numerous types, including localized amyloidosis, where production and deposition occur at the same site, and systemic amyloidosis, where amyloid forms in one site (commonly in the bone marrow) and deposits in different sites [9]. AL amyloidosis is the most prevalent form of the systemic type, characterized by the deposition of proteins derived from Ig LCs [10-12].
Commonly affected organs in AL amyloidosis include the heart, kidneys and liver, with GIT involvement being less common, and usually in the context of systemic disease. Typical symptoms of GIT involvement are fatigue, generalized weakness, and weight loss [13,14] but may also include constipation, delayed gastric emptying, abdominal distension, intestinal infarction, perforation, malabsorption and GIB [13,15,16]. GIB is observed in 12.5-36% of patients with AL amyloidosis with GIT involvement, although it is rarely the presenting symptom [7,14,16-18]. Our patient presented with multiple GIB events over 3 months, with no known plasma cell dyscrasia and no signs or symptoms of systemic disease.
Diagnosing amyloidosis involving the GIT is challenging because imaging and endoscopy findings are not specific, and biopsy of the affected tissue with specific histologic stains is necessary [19,20].
Common CT findings include bowel wall thickening, bowel wall dilatation without thickening, and, less commonly, mesenteric adenopathy and soft tissue infiltration [21]. As described in the literature, our patient presented with colon wall thickening and mesenteric lymphadenopathy on CTA, findings that are not specific to amyloidosis.
Frequent endoscopic findings in patients with amyloidosis and GIB are submucosal hematoma, mucosal ulceration and erosions, non-ulcerative inflammation, and submucosal lesions [7,22,23], although these are rarely seen in the colon [24]. Our patient was indeed presented with polypoid protrusions, submucosal masses, and bleeding markers located in the colon.
Despite undergoing right colectomy, our patient continued to experience subsequent episodes of GIB, and the pathology of the resected organ revealed diffuse rather than located or patchy, amyloid involvement. This discovery is not surprising since a previous study demonstrated that there is poor correlation between the site of amyloid deposition in the bowel and clinical manifestations [30].
All of these findings suggest that a complex mechanism is responsible for GIB in amyloidosis involving the GIT. Histologic findings in patients with GIB secondary to amyloid of the GIT suggest that infiltration of the vessel wall may lead to vascular fragility [25] and localized intestinal ischemia [6].
Another reported mechanism is infiltration of the intestinal wall, which leads to a less flexible muscularis mucosa that tends to tear [26]. These mechanisms, especially in patients with coagulation abnormalities such as prolongation of the prothrombin time (a frequent finding in systemic amyloidosis), increase the likelihood of GIB [27].
AL amyloidosis requires systemic treatment, and it is crucial to initiate prompt and appropriate treatment to reduce the disease burden and alleviate the complications resulting from specific organ involvement. The phase 3 ANDROMEDA trial [31], in which patients with AL amyloidosis received subcutaneous daratumumab a human IgG-κ monoclonal antibody that targets CD38, a glycoprotein uniformly expressed on human plasma cells, together with bortezomib, cyclophosphamide, and dexamethasone showed higher frequencies of hematologic complete response and survival free from major organ deterioration or hematologic progression, compared to the same regimen without daratumumab.
Our patient also showed remarkable response when daratumumab was added to bortezomib and dexamethasone, and achieved hematologic response after six months of treatment. While AL amyloidosis is usually a multi- organ process, our patient had isolated GIT involvement which responded to the administration of effective systemic treatment.
Conclusions
In summary, AL amyloidosis may manifest with unexplained recurrent GIB. It is crucial to consider this treatable cause in the differential diagnosis in such patients. The diagnosis is confirmed on Congo red staining of involved tissue which allows for specific and highly effective treatment.
References
- Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2019; 42-43: 101610.
- Kamboj AK, Hoversten P, Leggett CL. Upper gastrointestinal bleeding: etiologies and management. Mayo Clin Proc. 2019; 94: 697-703.
- Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003; 349: 583-96.
- Talar-Wojnarowska R, Jamroziak K. Intestinal amyloidosis: clinical manifestations and diagnostic challenge. Adv Clin Exp Med. 2021; 30: 563-70.
- Wechalekar AD, Gillmore JD. 25 years of systemic amyloidosis. International amyloidosis symposium, Groningen, Netherlands. 2012.
- Spier BJ, Einstein M, Johnson EA, Zuricik AO, Hu JL, Pfau PR. Amyloidosis presenting as lower gastrointestinal hemorrhage. Wis Med J. 2008; 107: 40-3.
- James DG, Zuckerman GR, Sayuk GS, Wang HL, Prakash C. Clinical recognition of AL type amyloidosis of the luminal gastrointestinal tract. Clin Gastroenterol Hepatol. 2007; 5: 582-8.
- Shirahama T, Cohen AS. High-resolution electron microscopic analysis of the amyloid fibril. J Cell Biol. 1967; 33: 679-708.
- Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011; 29: 1924-33.
- Nienhuis HLA, Bijzet J, Hazenberg BPC. The prevalence and management of systemic amyloidosis in Western countries. Kidney Dis (Basel). 2016; 2: 10-9.
- Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, et al. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2018; 25: 215-9.
- Simms RW, Prout MN, Cohen AS. The epidemiology of AL and AA amyloidosis. Baillieres Clin Rheumatol. 1994; 8: 627-34.
- Levy DJ, Franklin GO, Rosenthal WS. Gastrointestinal bleeding and amyloidosis. Am J Gastroenterol. 1982; 77: 422-6.
- Cowan AJ, Skinner M, Seldin DC, Berk JL, Lichtenstein DR, O’Hara CJ, et al. Amyloidosis of the gastrointestinal tract: A 13-year, single-center, referral experience. Haematologica. 2013; 98: 141-6.
- Yamada M, Hatakeyama S, Tsukagoshi H. Gastrointestinal amyloid deposition in AL (primary or myeloma-associated) and AA (secondary) amyloidosis: diagnostic value of gastric biopsy. Hum Pathol. 1985; 16: 1206-11.
- Madsen LG, Gimsing P, Schiødt FV. Primary (AL) amyloidosis with gastrointestinal involvement. Scand J Gastroenterol. 2009; 44: 708-11.
- Qi FX, Zhang Y, Ji YL, Jiang Y. Gastrointestinal manifestations of amyloidosis. World Chin J Digestology. 2019; 27: 260-6.
- Mumford AD, O’donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000; 110: 454-60.
- Tada S, Iida M, Iwashita A, Matsui T, Fuchigami T, Yamamoto T, et al. Endoscopic and biopsy findings of the upper digestive tract in patients with amyloidosis. Gastrointest Endosc. 1990; 36: 10-4.
- Bighi S, Trevisani L, Lupi L, Ariutti R, Cervi PM, Liboni A. Amyloidosis of the gastric stump: radiographic and CT findings. Gastrointest Radiol. 1990; 15: 197-8.
- Araoz PA, Batts KP, MacCarty RL. Amyloidosis of the alimentary canal: radiologic- pathologic correlation of CT findings. Abdom Imaging. 2000; 25: 38-44.
- Tada S, Iida M, Yao T, Kawakubo K, Yao T, Okada M, et al. Endoscopic features in amyloidosis of the small intestine: clinical and morphologic differences between chemical types of amyloid protein. Gastrointest Endosc. 1994; 40: 45-50.
- Chang HS, Myung SJ, Yang SK, Jung HY, Lee GH, Hong WS, et al. Massive small bowel bleeding in a patient with amyloidosis. Gastrointest Endosc. 2004; 59: 126-9.
- Iida T, Yamano H, Nakase H. Systemic amyloidosis with gastrointestinal involvement: diagnosis from endoscopic and histological views. J Gastroenterol Hepatol. 2018; 33: 583-90.
- Maeshima E, Yamada Y, Yukawa S. Massive gastrointestinal hemorrhage in a case of amyloidosis secondary to rheumatoid arthritis. Scand J Rheumatol. 1999; 28: 262-4.
- Kaiserling E, Kröber S. Massive intestinal hemorrhage associated with intestinal amyloidosis. An investigation of underlying pathologic processes. Gen Diagn Pathol. 1995; 141: 147-54.
- Yood RA, Skinner M, Rubinow A, Talarico L, Cohen AS. Bleeding manifestations in 100 patients with amyloidosis. JAMA. 1983; 249: 1322-4.
- Muchtar E, Dispenzieri A, Magen H, Grogan M, Mauermann M, McPhail ED, et al. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med. 2021; 289: 268-92.
- Tada S, Iida M, Yao T, Kawakubo K, Yao T, Fuchigami T, et al. Gastrointestinal amyloidosis: radiologic features by chemical types. Radiology. 1994; 190: 37-42.
- Petre S, Shah IA, Gilani N. Review article: gastrointestinal amyloidosis - Clinical features, diagnosis and therapy. Aliment Pharmacol Ther. 2008; 27: 1006-16.
- Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, et al. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis. N Engl J Med. 2021; 385: 46-58.