
Case Report
Ann Hematol Onco. 2024; 11(1): 1445.
Posterior Reversible Encephalopathy Syndrome in an Elderly Patient with Acute Myeloid Leukemia
Stephen Lee Yu, MD, MS¹; Vishal Deepak, MD²; Salah Ud Din Safi, MD, MS¹; Rahul Sangani, MD³; Sarah Hadique, MD³
1West Virginia University Section of Hematology and Oncology, Department of Internal Medicine, USA
2Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, New York University Langone Health, New York, USA
3West Virginia University, Section of Pulmonary Critical Care and Sleep Medicine, Department of Internal Medicine, USA
*Corresponding author: Stephen Lee Yu, MD, MS 64 Medical Center Drive, HSC, Division of Hematology/Oncology, PO Box 9162, 3rd Floor, Cancer Center, Rm 3911A, Morgantown, WV 26506, USA. Email: Stephen.yu@hsc.wvu.edu
Received: December 18, 2023 Accepted: January 12, 2024 Published: January 19, 2024
Abstract
Posterior Reversible Encephalopathy Syndrome (PRES) is a neurological condition presenting with encephalopathy, seizures and headache with concurrent edema in the posterior cerebral circulation. While hypertension is the most common associated, PRES has been reported in patients receiving chemotherapy for various cancers such as AML. We report a 61-year-old female with history of hypertension who presented with sepsis and a new diagnosis of Acute Myeloid Leukemia (AML). She was started on broad spectrum antibiotics for her sepsis concurrently with Azacitidine and Venetoclax for treatment of sepsis and AML, respectively. Shortly after initiation of her AML treatment, patient became encephalopathic and experienced tonic clonic seizures. She had clinical and imaging features consistent with PRES. PRES has been reported as rare complication of chemotherapy of various cancer generally, and AML specifically. It is important to recognize it in a timely manner to prevent further damage. This case report highlights this important complication of the chemotherapy initiation in AML especially in elderly patient who presented with sepsis.
Keywords: AML (Acute Myeloid Leukemia), PRES (Posterior reversible encephalopathy syndrome), case report, Azacitidine and Venetoclax
Abbreviations: AML: Acute Myeloid Leukemia; PRES: Posterior Reversible Encephalopathy Syndrome; CT: Computed Tomography; MR: Magnetic Resonance; MICU: Medical Intensive Care Unit
Introduction
Treatment of newly diagnosed Acute Myeloid Leukemia (AML) comes with myriad of different adverse effects based on the treatment regimen. This becomes especially important in advanced age population who often have poor tolerance to standard high-intensity chemotherapeutic agents. Azacitidine in combination with Venetoclax have shown good safety profile and well tolerated by elderly patients with AML, and even shown favorable overall response rate in untreated elderly AML patients [1,2]. While the safe profile in Azacitidine in combination with Venetoclax make it an optimal treatment of choice for advanced age population, post-marketing to look for further adverse effect is of critical importance. One of the rare side effect of treatments of AML is Posterior Reversible Encephalopathy Syndrome (PRES), which is a neurological condition characterized by headache, visual changes, seizures, and encephalopathy with concurrent symmetrical edema predominant in the posterior cerebral region seen in the Computed Tomography (CT) and Magnetic Resonance (MR) imaging [3]. To our knowledge, there has been one case report reported in two patients with AML, who developed PRES during induction chemotherapy [4]. Here, we present a case of elderly patient who have started treatment for newly diagnosed AML and developed PRES during treatment initiation with Azacytadine and Venetoclax.
Case Presentation
A 61-year-old female with history of hypertension presented to emergency department with complains of progressive fatigue, dyspnea, fever, hemoptysis, and increased bruising. Her vital signs at the time of presentation were within normal range and physical examination was notable for dried blood in both nares.
Laboratory findings at the time of presentation showed pancytopenia with white blood cell count of 0.9 x 103/μL, hemoglobin of 5.8 g/dL, and platelets of 5 x 103/μL. She was also noted to have mass like areas of similar size in the bilateral upper lobes concerning for atypical pneumonia on chest x-ray. Further evaluation with a Computed Tomography (CT) chest showed ill-defined alveolar density in the bilateral upper lobes, more pronounced near the left apex concerning for multifocal pneumonia with underlying pulmonary mass at the lung apices (Figure 1).
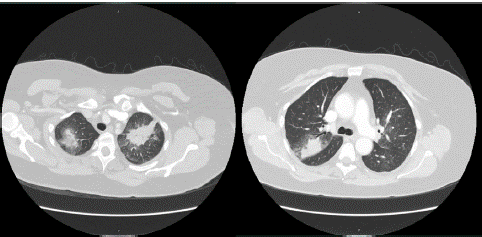
Figure 1: Axial cuts at different levels of CT chest with contrast showing ill-defined alveolar densities in both upper lobes with most pronounced in the left lung apex.
Patient was admitted to the hospital and received multiple units of blood products in setting of her low cell counts and epistaxis. She was also started on broad spectrum antibiotics for neutropenic fever and pneumonia. Of note, blood cultures from the time of presentation eventually grew Staphylococcus epidermidis in two out of the four bottles.
On further work up, she was noted to have blast cells in addition to pancytopenia followed by flow cytometry which showed increased myeloblast population. She underwent a bone marrow biopsy which confirmed the diagnoses with AML with presence of 68% myeloblast population. Patient was transferred to a tertiary care hospital for further work up and treatment for AML.
At the tertiary care center, patient had a repeat bone marrow biopsy which was sent for next generation sequencing, and fluorescent in situ hybridization analysis. Next generation sequencing was positive for ASXL1, IDH1, PHF6, TET2, and U2AF1 mutation with normal cytogenetics. Human leukocyte antigen typing was also completed for possible transplant evaluation in the future. She was started on Azacitidine 75 mg/m2 daily for 7 days every 28 days and Venetoclax 100 mg daily for treatment of AML while broad spectrum antibiotics were continued. Patient’s hospitalization course was complicated by continued pancytopenia, epistaxis and worsening pneumonia on interval CT chest. Epistaxis was addressed by otolaryngologist with nasal packing.
On hospital day 18, after cycle 1 day 15 on Azacitidine and Venetoclax, patient experienced generalized tonic-clonic movements which resolved with 1 mg of Lorazepam. She was intubated for airway protection due to continued unresponsiveness and was transferred to Medical Iintensive Care Unit (MICU). After arrival to MICU, patient was noted to be persistently encephalopathic. CT brain done after seizure-like episode showed asymmetric white matter edema on the bilateral occipital lobes, raising concern for PRES. Electroencephalogram performed on the day of witness seizures showed cerebral encephalopathy without epileptiform discharges. Further imaging with MR imaging of the brain showed a patchy area of subcortical white matter vasogenic edema to the posterior parietal and occipital lobes (Figure 2). In the context of new onset encephalopathy and imaging findings, diagnosis of PRES was established.
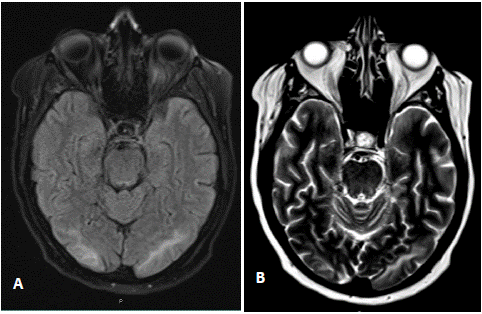
Figure 2: Axial view of FLAIR (a) and T2-weighted (b) MR imaging of the brain without contrast showing patchy area of subcortical white matter vasogenic edema to the posterior parietal and occipital lobes.
Patient’s AML treatment was held in setting of worsening clinical status, and she was started on intravenous nicardipine infusion to strictly maintain blood pressure in normal range. Her encephalopathy gradually improved after discontinuation of chemotherapy and supportive measures. While in the MICU, a flexible bronchoscopy was performed, and analysis of bronchoalveolar lavage fluid from right and left upper lobe was negative for typical and atypical infections. She was eventually extubated on hospitalization day 21. However, due to significantly worsening performance status, patient was deemed not a candidate for further chemotherapy. Goal of care discussion for the patient were held with patient’s family, who elected to discharge the patient home with hospice.
Discussion and Conclusion
PRES is a reversible neurological manifestation most commonly precipitated by elevated blood pressures, renal damage, hypervolemic state, and patients who are treated with immunosuppressive agents [3,5]. There are multiple reported cases in pediatric settings where PRES have been seen during treatment of hematological malignancies with chemotherapy including, acute lymphoblastic leukemia, AML, chronic myeloid leukemia, and non-Hodgkin lymphoma [6-8]. In a retrospective chart review of adult population at a large cancer institute showed that PRES was diagnosed more in women, who had undergone treatment with chemotherapy or a biologic agent. Of the patients who were diagnosed with PRES, majority received therapies within 30 days preceding diagnosis of PRES. In our patient, PRES was diagnosed within 30 days of initiation of chemotherapy [9]. Based on the risk factors and temporal relation of the symptoms with chemotherapy, we concluded that the PRES was related to the treatment for AML. Moreover, patient’s encephalopathy improved after discontinuation of chemotherapeutic agents and other supportive measures further supporting this thought process.
In a systemic literature review of 70 cases by How and her colleagues, most common chemotherapy associated with PRES included platinum-containing drugs, cyclophosphamide, hydroxydaunorubicin, vincristine, R-CHOP and gemcitabine. Hypertension was the most common risk factor for development of PRES after chemotherapy [11]. In our patient’s case, the patient developed PRES during chemotherapy with demethylating agent (Azacitidine) and a small molecule B-cell lymphoma-2 inhibitor (Venetoclax). PRES has never been reported with these chemotherapy agents. However, grand mal convulsion has been reported as post market adverse reactions from Azacitidine [12]. To the best of our understanding, to date, Venetoclax does not have any neurotoxicity reported in large cohort studies or in post-marketing analysis. There are multiple theories proposed for the pathogenesis of PRES including vasogenic, endothelial and cytotoxic theory [13]. Out of the proposed mechanisms for PRES, likely mechanism for the patient in this case report is endothelial dysfunction from the cytotoxic effects from chemokines during the treatment of AML by chemotherapy and underlying sepsis.
In respect to PRES associated with AML, Battipaglia et al., described two cases to PRES after induction chemotherapy for AML in a letter to editor. [4] To our knowledge these are the only reported cases of AML developing PRES after induction chemotherapy. In contrast to the above reported patient, our patient was slightly different as she did not receive induction therapy rather a combination of above-mentioned agents. Furthermore, our patient was relatively older as well.
This is the second report of PRES in adult with AML that has occurred in setting of treatment initiation and infection, but the first case report of PRES reported in an advanced age demographic. As such, it is imperative to quickly recognize the symptoms and presentation of these neurological manifestations and treated promptly to avoid further harm.
Author Statements
Acknowledgements
We appreciate Dr. Joe Joseph, MD, an assistant professor in neuroradiology at West Virginia University Neuroradiology department, for reviewing and assisting with imaging selection for this case report.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Funding
No fundings were provided for production from the conception and finalization of this case report.
Authors’ Contributions
SLY and VD has contributed majority of the written products from its conception to the finalized document until submission. SS provided expertise in hematological perspective in this case and was a major contributor in writing the case report. SH and RS has provided conception of the work described in this case and provided substantial amount of contribution in writing the case report.
References
- DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019; 133: 7-17.
- DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020; 383: 617-29.
- Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 334: 494-500.
- Battipaglia G, Avilia S, Morelli E, Caranci F, Perna F, Camera A. Posterior reversible encephalopathy syndrome (PRES) during induction chemotherapy for acute myeloblastic leukemia (AML). Ann Hematol. 2012; 91: 1327-8.
- Sudulagunta SR, Sodalagunta MB, Kumbhat M, Settikere Nataraju A. Posterior reversible encephalopathy syndrome (PRES). Oxf Med Case Rep. 2017; 2017: omx011.
- Lucchini G, Grioni D, Colombini A, Contri M, De Grandi C, Rovelli A, et al. Encephalopathy syndrome in children with hemato-oncological disorders is not always posterior and reversible. Pediatr Blood Cancer. 2008; 51: 629-33.
- Danhofer P, Tomečková M, Černá D, Zapletalová D, Horák O, Aulická Š, et al. Prognostic factors and seizure outcome in posterior reversible encephalopathy syndrome (PRES) in children with hematological malignancies and bone marrow failure: A retrospective monocentric study. Seizure. 2019; 72: 1-10.
- Appachu MS, Purohit S, Lakshmaiah KC, Kumari BS, Appaji L. Posterior reversible encephalopathy syndrome in pediatric acute leukemia: case series and literature review. Indian J Med Paediatr Oncol. 2014; 35: 79-82.
- Singer S, Grommes C, Reiner AS, Rosenblum MK, DeAngelis LM. Posterior reversible encephalopathy syndrome in patients with cancer. Oncologist. 2015; 20: 806-11.
- Marjoncu D, Andrick B. Gilteritinib: A novel FLT3 inhibitor for relapsed/refractory acute myeloid leukemia. J Adv Pract Oncol. 2020; 11: 104-8.
- How J, Blattner M, Fowler S, Wang-Gillam A, Schindler SE. Chemotherapy-associated posterior reversible encephalopathy syndrome: A case report and review of the literature. Neurologist. 2016; 21: 112-7.
- Corportaion C. Vidaza azacitidine for injection, Product Monograph including patient medication information. Celgene. 2019. Available from: https://www.bms.com/assets/bms/ca/documents/productmonograph/VIDAZA_EN_PM.pdf.
- Gandini J, Manto M, Charette N. Delayed posterior reversible leukoencephalopathy syndrome triggered by FLOT chemotherapy. Front Neurol. 2019; 10: 1405.