
Research Article
Ann Hematol Onco. 2024; 11(2): 1450.
A Survey on Quality-of-life Indicators During the Last Months of Terminally ill Cancer Patients in Iran; A Cross-Sectional Study in a Home-based Palliative Care
Heshmatolah Heydari1,9; Kosar Sadat Hosseini Kolbadi2; Suzanne Hojjat-Assari3; Vahid Kaveh4; Maryam Vahabi5; Mahdi Rezai6; Naeemeh Keshavarzi7; Ghazal Razan
1PhD in Nursing, Associate Professor, Social Determinants of Health Research Center, School of Nursing and midwifery, Lorestan University of Medical Sciences, Khorramabad, Iran
2Medical Student, Student Research Committee, Iran University of Medical Sciences, Tehran, Iran
3Specialist in Immunohematology, French Institute of Research and High Education (IFRES-INT), Paris, France
4Department of Medical Oncology and Hematology, Iran University of medical Sciences, Tehran, Iran
5Industrial Engineering Department, Iran University of Sciences and Technology, Tehran, Iran
6Emergency Medicine Management Research Center, Iran University of medical Sciences. Tehran. Iran
7General Physician in the Palliative Care, Department of Home-Based Palliative Care, ALA Cancer Prevention and Control Center (MACSA), Tehran, Iran
8Medical student. Student Research Committee, Iran University of Medical Sciences, Arak, Iran
9French Institute of Research and High Education (IFRES-INT), Paris, France
*Corresponding author: Kosar Sadat Hosseini Kolbadi, General Practitioner, University of Medical Sciences, Tehran, Iran. Email: kosar.hossinie@gmail.com
Received: March 18, 2024 Accepted: April 26, 2024 Published: May 03, 2024
Abstract
Background: Frequent referring to hospitals, recurrent and long-term hospitalizations are the factors reducing the quality-of-life of patients during the palliative phase in the cancer patients. Therefore, this study aimed to determine quality-of-life indicators during the last months of terminally ill cancer patients.
Methods: This retrospective cross-sectional study was performed on 371 patients with advanced end-stage cancer referring to the MACSA Home Care Center from March 21, 2018 to September 22, 2018 for receiving palliative care and support. The information was gathered from the patients’ files by a checklist. Two indicators were considered to evaluate the patients’ quality of last months of life. All data were analyzed using Minitab software version 19 using descriptive and inferential statistics.
Results: The quality index of end-of-life months based on the mean hospitalization length during the last months of life was 9.3% for the patients who had received at-home care at least once and 33.8% for those who died in the hospital. The end-life quality index was 12.4% for the patients who had received at-home care at least once and were hospitalized more than once during the last months of life and 41.8% for those who died in the hospital. The life quality index of the mean number of inpatient nights was higher in those who died in the hospital (10.1).
Conclusion: It seems necessary to provide at-home palliative care infrastructure in order to improve the quality-of-life indicators of patients with advanced cancer during the last months of their lives.
Keywords: Palliative care; Quality-of-life Indicators; Advanced cancer; Terminally ill; Home health care
Background
The International Agency for Research on Cancer has reported the 2021 global incidence of cancer as 19.3 million and its related mortality as 10 million [1]. Cancer is predicted to be the leading cause of death in the world by 2030 [2]. About 60-70% of cancer-related deaths occur in low- and middle-income countries [3]. In recent years, with the growth of industrialization of Iran and changes in people’s lifestyles, the epidemiological trends of malignancies have changed [4,5]. Cancer is known as the second leading cause of death in Iran [6]. In 2018, 110,115 new cases of cancer have been identified in Iran, and 55,785 cancer-related deaths have been reported in the same year [2].Patients with incurable cancers face complicated problems in the later stages of their lives. Physical problems and medical emergencies lead to frequent visits to hospitals and long-term hospitalizations, occupying hospital beds [7]. The lack of necessary managerial structures for handling the last months of life of these patients not only imposes heavy financial burdens on the health system but also reduces the quality of life of the patient and his/her family [8,9]. The time spent in the hospital during the last months of life of cancer patients is one of the quality indicators for palliative care. This index is below 10% in some developed countries [10].
Chemotherapy is one of the therapeutic approaches in cancer. Chemotherapy in the last 14 days of life is regarded as an invasive, unnecessary, and costly procedure [11,12], however, it may be used in patients with metastatic cancer to prevent disease progression and extend the patient’s life expectancy [13]. Deciding on the necessity of palliative chemotherapy depends on the risk/benefit assessment for the patient. Patients and oncologists face difficult decisions with regard to chemotherapy during the palliative phase. Although treatment may prolong survival or mitigate symptoms, it may also accompany side effects [14,15].
The World Health Organization (WHO) has recognized palliative care as a way to promote the quality-of-life of end-stage cancer patients [16]. The approach of palliative care during the end stages of life is to help the medical team refuse demands for invasive treatments and prepare the patient and his/her family for the last days of the patient’s life. This type of care entails a comprehensive view on the patient’s situation and aims to reduce undesirable physical complications, fulfil the patient’s psychological, spiritual, and social needs, and increase the quality-of-life of the patient and his/her family [2]. This type of care not only helps patients live an active and dynamic life until death, but also supports patients’ families during the disease course, at the time of death, and after death, making them accept the event more peacefully [16]. The place of service provision matters when it comes to providing optimal services to these patients. Palliative care can be provided to patients in hospitals, special clinics, hospices, or at homes, among which the latter is the least expensive and often most appropriate from the perspective of patients and families [17-21]. Home-based palliative care can increase the quality of life of patients and caregivers, shorten the length of hospitalization, less referrals to emergency wards and higher rates of death at home [22,23]
Iran’s health sector follows a level-based referral system [24]; however, home-based palliative care, as a new care provision approach, has no place in this structure. Therefore, home-based palliative care services are provided to populations by private and charitable centers [25]. A non-for-profit organization, Iranian Cancer Control Center (MACSA), has been the primary and largest provider of specialized palliative care services to cancer patients in Iran since 2007. The center provides services to patients at home or in the hospital, and its hospital-based sector is located in Firoozgar Hospital of Tehran, affiliated with Iran University of Medical Sciences. The services provided by MACSA centers include medical, nursing, rehabilitation, and counseling (spiritual, psychological, and nutritional) services, as well as social work.
There is little information about the frequency of visits to clinics, hospitalization, and the use of chemotherapy during the palliative phase in Iranian patients with advanced cancer. So, conducting a study in this regard can help delineate the current situation so that experts and policymakers can arrange appropriate plans for cancer patients in their later stages of life. Therefore, this cross-sectional study was performed to determine the quality of end-of-life months of Iranian patients with advanced cancer receiving home-based palliative care.
Methods
This retrospective cross-sectional study was performed on patients with advanced cancer who referred to the MACSA Home Care Center from March 21, 2018 to September 22, 2018 to receive palliative care and support.
Inclusion criteria were an age of at least 18 years old, suffering from cancer, being a resident of Tehran, and registration at the MACSA Home Care Center for receiving home care services during the study period. Exclusion criteria included moving to a new place during the study, withdrawal from receiving palliative care, and incomplete patient records. Sampling was conducted through the census using a checklist. The data collection tool was designed based on the information available in patients’ files (at the home care center and the referral hospital) and expert opinions. Two (one four-year and one fifth-year) medical students were assigned for data collection. First, a list of all patients registered at MACSA during the study period was prepared, and the reasons for their referrals were also recorded. Out of this list, the patients who had received at least one episode of home care were categorized into group A. The patients of group A were further subcategorized into either group B (no hospital visit) and group C (at least one hospital visit).
The patients of group C were further divided into either group D (receiving outpatient counseling in the hospital) or group E (hospitalization for at least one night). Finally, the patients of group E were classified into either group F (no chemotherapy during hospitalization) or group G (receiving at least one episode of chemotherapy during hospitalization) (Figure 1).
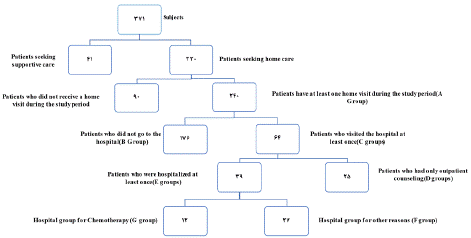
Figure 1: Subjects of study.
Based on the checklist prepared, demographic data (gender, age), pathological diagnosis, the reasons for referral to the hospital and receiving home care, and the information related to death were gathered. The data related to the hospital visit included the number of outpatient consultations received, the length and frequency of hospitalizations, and the episodes of chemotherapy. In addition, the data related to home-based care included the number of medical and nursing visits and the number of psychological counseling received. Then the patient’s date of death was extracted from the files, and the time intervals from admission and the first hospitalization to death were recorded. These data were related to a period up to one year after enrollment.
Two indicators were used to evaluate the quality of patients’ last months of life. The first indicator was the time spent by patients in the hospital during their last months of life, and the second indicator was the frequency of hospitalization during last months of life. After being extracted from the paper and electronic files of patients at MACSA Home Care Center and Firoozgar Hospital, these data were entered into the pre-prepared checklists. In the case of ambiguity, phone calls were made to the patients’ families or physicians to complete data. All data were entered into and analyzed by Minitab software version 19 using relevant descriptive and inferential statistics. Central tendency and dispersion indices, including mean, standard deviation, median, minimum, and maximum, were determined for all variables.
Ethical Considerations
All ethical considerations were tried to be observed. All patients at the time of registration at the MACSA center gave written consent to using their information in this research. All data were kept confidential and used only for research purposes in this study. This study received ethical approval from the ethics committee of Iran University of Medical Sciences with Cod: IR.IUMS.REC.1400.243.
Results
Data analysis showed that out of 371 patients who registered at MACSA during the study period, 330 patients requested the receiving of home-based services, and the rest wished to receive supportive services (specialized counseling, medical equipment, etc.). Of the patients applying for home services, 240 were visited at home at least once (group A). Other patients were excluded from the study due to having at least one of the exclusion criteria. Among the patients of group, A, 64 visited the referral hospital at least once (group C); 39 of whom were hospitalized for at least one night (group E) while the remaining 25 patients only received outpatient counseling after referral to the hospital (group D). Out of 39 patients with a history of hospitalization (i.e., group E), 12 had received chemotherapy at least once (group G) while others (n=27) did not receive chemotherapy during hospitalization (group F) (Figure 1).
Most of the patients were male (53.75%) with a mean age of 64.9 ± 12.8 years. Gastrointestinal cancers constituted the most common types of cancer (47.9%) (Table 1).
Sex
Age
Frequency of cancer
Frequency of Received Home Care
Group
Frequency of Patients
Female, NO (%)
Male, NO (%)
Year, M±SD
GI Tract NO (%)
Urogenital System, NO (%)
Respiratory System
Hematologic
Others
Unknown
Medical and Nursing
Frequency
Mean
A
240
111(46.25%)
129(53.75%)
64.9±12.8
115 (47.9%)
37 (15.4%)
24 (10%)
10 (4.2%)
16.70%
5.80%
1137
4.7
A
B
176
85
91
66.5±12.7
-
-
-
-
-
-
-
-
C
64
26
38
60.3±12.1
-
-
-
-
-
-
-
-
C
D
25
10
15
60.6±11.5
11 (44.0%)
4 (16.0%)
4 (16.0%)
3 (12.0%)
12.00%
0.00%
128
5.3
E
39
16
23
60.1±12.6
-
-
-
-
-
-
161
4.1
E
F
27
9
18
60±13.3
12 (44.4%)
9 (33.3%)
2 (7.4%)
2 (7.4%)
3.70%
3.70%
90
3.3
G
12
7
5
60.3±11.7
5 (41.7%)
2 (16.7%)
1 (8.3%)
0 (0.0%)
33.30%
0.00%
71
5.9
GI Tract: Gastrointestinal Tract; NO: Number.
Table 1: Frequency of Subjects according to sex, age and type of cancer.
Data analysis showed that the mean number of hospital visits was higher in group E than in group D; however, the difference was not statistically significant (90% CI, P-value = 0.55). Also, the mean number of hospital visits was higher in group G than in group F, which was statistically significant (90% CI, P-value = 0.08). The mean number of hospitalization episodes (90% CI, P-value = 0.001) and the mean length of hospitalization (90% CI, P-value = 0.002) were significantly higher in group G compared to group F. In the patients of group G, hospitalization due to chemotherapy was significantly more frequent than other reasons (90% CI, P-value = 0.02), but there was no significant difference between these two groups of patients (i.e., admission due to chemotherapy vs. other reasons) in terms of the mean number of hospital admission nights (90% CI, P-value = 0.25, Table 2).
Type of service
Groups
Frequency of Hospitalization
M ±SD of Hospitalizations
P-value
Outpatient counseling
D
25
78
3.1±4
0.55
E
39
177
4.5±7.1
E
F
27
76
2.8±3.9
0.08
G
12
101
8.4±10.7
Hospitalization services
Frequency of hospitalization
F
27
38
1.4±1
0.001
G
12
77
6.4±5.6
Overnight hospitalization
F
27
126
5±5.5
0.002
G
12
172
14.3±9.7
Hospitalization services of group G
Frequency of hospitalizations
For chemotherapy
G
12
57
4.7±4.4
0.02
Other reasons
G
12
20
1.7±2.2
Overnight hospitalizations
For chemotherapy
G
12
95
7.9±5.6
0.25
Other reasons
G
12
77
6.4±6.6
Table 2: Frequency of receiving outpatient counseling and inpatient services in different groups.
Data analysis showed that among 21 patients in group F, 81% died during the first six months after the first hospital admission. Regarding the location of death, most patients of group F (70.8%) died at home while most patients of group G (62.5%) died in the hospital. Among seven patients in group G, the rate of one-year survival from the first hospital admission to death was 33.3% (Table 3).
Groups
Frequency
Evaluation start time
Death time (survival rate)
Frequency of deaths in each group
Death location
0-6 Months NO (%)
6-12 Months NO (%)
Alive for more than 1 year NO (%)
Unknown
Hospital
NO (%)Home
NO (%)Unknown
A
240
Since filing
77.9%
1879.2%
2211.7%
281.2%
212
38.2%
8161.3%
1300.5%
D
25
Since filing
16(64%)
12%
320%
54%
20
55%
1145%
90.0%
E
F
27
Since filing
74.1%
2011.1%
311.1%
33.7%
24
29.2%
770.8%
170.0%
First Hospitalization
81.5%
223.7%
111.1%
33.7%
24
G
12
Since filing
41.7%
525%
333.3%
40.0%
8
62.5%
537.5%
30.0%
First Hospitalization
58.3%
78.3%
133.3%
40.0%
8
Table 3: Comparison of the distribution of survival rates in different groups from the time of filing for one year.
The quality index of end-of-life months based on the mean duration (nights) of hospitalization was obtained 9.3% for the patients who received at least one at-home visit (i.e., group A) and 33.8% for those who died in the hospital. Also, the quality index of end-of-life months was recorded 12.4% for the patients receiving at least one at-home visit (i.e., group A) and were hospitalized more than once in the last months of life and 41.8% for those who died in the hospital. The mean number of hospital admission nights was higher in the patients who died in the hospital (10.1) compared to that (2.8) of all participants. Also, the index of the mean number of hospitalization episodes during the last months of life was higher in the patients who died in the hospital (41.8%) compared to that (12.4%) of all participants (Table 4).
Index of time spent in hospital in the last month of life
Hospitalization index more than once in the last month of life
Nights of hospitalization in the last month
Unknown
Index (%)
Number of people with more than one hospitalization
Unknown
Index (%)
Frequency
Mean
All subjects in A group
609
2.8
21
9.3%
28
14
12.4%
A groups subjects dead in hospital
609
10.1
21
33.8%
28
14
41.8%
Table 4: End of life indicators.
Discussion
This study was conducted to evaluate the quality of the end-life months of patients with advanced cancer referring to a palliative home care provider center in Iran. The results showed that the number of referrals to the hospital, hospital admission episodes, the length of hospitalization, and the use of chemotherapy were low for all patients during their last months of life. The data also showed that the use of chemotherapy increased all of the indicators mentioned, leading to a reduction in quality of life.
Data revealed that 27.3% of the patients visited the hospital at least once. The number of outpatient visits was higher for the patients who had the history of at least one night hospitalization compared to those who merely referred for outpatient counseling. Another study reported that a large number of cancer patients referred to the hospital during the last six months of their lives [26], which is higher than the rate reported in this study. The number of hospital admissions during the last months of life is a quality indicator for palliative care provision and for the assessment of the patient’s quality of life [10,26]. Although hospitalization during the last months of life may be associated with invasive procedures and futile treatments that reduce the patient’s quality of life (7, 26), hospital admission in the terminal phase is sometimes inevitable [27]. So, health systems should provide appropriate facilities for these patients and in emergency situations.
The findings of this study showed that the average number of hospitalizations and the mean duration (nights) of hospital stay were higher in the patients who received chemotherapy in the hospital than those who either received outpatient care or were hospitalized for reasons other than chemotherapy. In line with the findings of this study, another study reported that the quality-of-life index during the last months of life was better for the patients who received home-based rather than hospital-based care [28]. The number of hospitalizations and the duration (the number of nights) of hospital admission reported in this study were higher compared to those described in other studies, which could be due to reasons such as health system structure, various models of service delivery to cancer patients, and the cultural beliefs of patients and health care providers. The number of hospitalizations alone cannot be a proper quality-of-life indicator during the last months of life, but when it is integrated with the index of the time spent in the hospital during the last month of life, a more comprehensive view can be obtained on the quality of palliative care services. The findings of the recent study also showed that the receiving of palliative care services through the network of general practitioners resulted in more satisfactory outcomes in terms of both of these quality-of-life indicators [7].
Data showed that 5% of the patients studied received chemotherapy in the last months of their lives, which agreed with the findings of Van Baal et al. who reported that 10.4% of their patients underwent chemotherapy during the last month of their lives (28). Other studies have reported that 20-50% of cancer patients undergo chemotherapy in their last months to improve their quality of lives and survival [29-31]. The rate reported in this research is lower compared to that described by other studies, which may be attributable, at least in part, to the positive role of at-home palliative care in reducing hospital visits and the use of chemotherapy.
Chemotherapy in patients with metastatic cancer aims to prolong life expectancy; however, during the palliative phase, the purpose of this procedure is to maintain patients’ quality of life and reduce the complications of the disease [29]. In recent years, the development of novel anticancer drugs with less side effects and greater effectiveness has increased the use of chemotherapy during the palliative phase, improving the life expectancy of patients and modifying the criteria of using invasive treatments for cancer [14,29]. Given that chemotherapy in the last months of life compromises the quality of life of patients [30,32,33], health systems should consider measures to properly manage the use of this approach during the last days of patients with end-stage cancer.
The findings of this study showed that among the patients admitted to the hospital, the frequency of referrals and the length of hospitalization were higher in those who received chemotherapy during admission. The data also showed that only 33.3% of the patients undergoing chemotherapy during hospitalization survived beyond one year after the first admission compared to the patients who did not receive chemotherapy.
In the present study, although the exact indications for palliative chemotherapy were not explicitly explained in the patients’ records, it seems that alleviation of the symptoms, improvement of the patient’s physical condition before starting home care, the family’s demands have been the main reasons for the decision. In some cases, despite the witnessing of the ineffectiveness of anti-cancer therapies, the families would still persistently ask for more invasive treatments just to postpone the inevitable death [15,34]. The start of palliative care requires a combination of economic, cultural, social, and political issues [35]. The treatment team should inform the patient and the family (i.e., family care) about the treatment process and indications for the termination of curative treatments and the beginning of palliative care [36]. Holding informative sessions with the patient and the family at the onset of palliative care (i.e., family care) can help prepare them for the difficult days ahead. In family counseling sessions during palliative care, one important axis should be the designation of a competent individual as the definite decision-maker.
Although some studies have reported that the use of palliative chemotherapy may increase the risk of mortality in patients [27], authors in this study did not observe any evidence suggesting a reduction in life expectancy due to chemotherapy. This state might have been influenced by the low number of the patients who underwent chemotherapy. Our results showed that less frequent hospitalizations could increase patients’ quality of life. In this regard, Westergaard et al. reported that 70% of cancer patients visited the hospital during the last six months of their lives [7]. Numerous studies have considered the number of hospitalizations in the last months of life as a quality indicator for palliative care provision or as an index for the patient’s quality of life [7,26].
Limitations
This was a cross-sectional study in a single center for the provision of home-based palliative care and a referral hospital. For various reasons, our sample population might have been restricted to a specific class of the society, which might have predisposed the results to sampling bias. So, it is recommended to conduct a similar comprehensive study in more centers. At first, we expected a survey on the patient data from one full year; however, due to the Covid-19 outbreak and restrictions in access to the data, the analysis was performed only on the data from the first half of 2019. Finally, data was obtained retrospectively by reviewing patient records, and the lack of complete data in some files was another limitation of this study.
Conclusion
Our results showed that the frequency of referrals to the hospital, frequency of hospitalization, the length of hospitalization, and the administration of palliative chemotherapy were lower in this study compared to similar reports, which can be partly related to the accessibility of these patients to advanced palliative care. Therefore, it is advisable to provide the necessary infrastructure required for establishing home-based palliative care for cancer patients during end-stage life. Also, palliative chemotherapy in the last months of life can lead to more outpatient visits or hospital admissions while it does not have a significant impact on patient survival. Thus, it seems necessary to develop appropriate guidelines to manage the treatment process in these patients and scientifically decide whether patients should receive chemotherapy or not.
Author Statements
Ethics Approval and Consent to Participate
All methods were performed in accordance with the relevant guidelines and regulations by in the declaration of Helsinki (ethics approval and consent to participate). This study was approved at the Ethics Committee at Iran University of medical sciences “IR.IUMS.REC.1400.243”. The investigators considered ethical principles of obtaining written consent form participants, confidentiality of data, and anonymity of participants in all phases of the study.
Availability of Data and Material
This study conducted by Quantitative method, audience and text file are available from the corresponding author if needed.
Competing Interests
The authors declare that they have no competing interests.
Funding
This study was not financially supported by any organization.
Author's Contributions
SA: Investigator; parti cipated in study design, data collection, data analysis, accrual of study participants, manuscript writing and review. KH, GhR, Vk and NK: Investigators; participated in study design, data collection, manuscript writing and review. MV: Investigator; participated in study design, data analysis, manuscript writing and review. HH: Investigator; participated in study design, data collection, data analysis, accrual of study participants, manuscript writing and review. All authors read and approved the final version of the manuscript.
Acknowledgements
Authors would like to profusely thank all individuals who supported and helped them to conduct this study.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021; 71: 209-49.
- Dolatkhah R, Somi MH, Kermani IA, Ghojazadeh M, Jafarabadi MA, Farassati F, et al. Increased colorectal cancer incidence in Iran: a systematic review and meta-analysis. BMC public health. 2015; 15: 1-14.
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today. Lyon: International Agency for Research on Cancer. 2018. 2020.
- Salim EI, Moore MA, Al-Lawati JA, Al-Sayyad J, Bazawir A, Bener A, et al. Cancer epidemiology and control in the arab world-past, present and future. Asian Pac J Cancer Prev. 2009; 10: 3-16.
- Almasi Z, Rafiemanesh H, Salehiniya H. Epidemiology characteristics and trends of incidence and morphology of stomach cancer in Iran. Asian Pacific journal of cancer prevention. 2015; 16: 2757-61.
- World Health Organisation. Profile of Noncommunicable Diseases in Iran, 2018. 2021.
- Vestergaard AHS, Neergaard MA, Christiansen CF, Nielsen H, Lyngaa T, Laut KG, et al. Hospitalisation at the end of life among cancer and non-cancer patients in Denmark: a nationwide register-based cohort study. BMJ open. 2020; 10: e033493.
- Mehlis K, Witte J, Surmann B, Kudlich M, Apostolidis L, Walther J, et al. The patient-level effect of the cost of Cancer care – financial burden in German Cancer patients. BMC Cancer. 2020; 20: 529.
- Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial Hardships Experienced by Cancer Survivors: A Systematic Review. JNCI: Journal of the National Cancer Institute. 2016; 109.
- van Baal K, Schrader S, Schneider N, Wiese B, Stahmeyer JT, Eberhard S, et al. Quality indicators for the evaluation of end-of-life care in Germany – a retrospective cross-sectional analysis of statutory health insurance data. BMC Palliative Care. 2020; 19: 187.
- Zhu Y, Tang K, Zhao F, Zang Y, Wang X, Li Z, et al. End-of-life chemotherapy is associated with poor survival and aggressive care in patients with small cell lung cancer. Journal of cancer research and clinical oncology. 2018; 144: 1591-9.
- Zhang Z, Chen M-L, Gu X-L, Liu M-H, Zhao W-W, Cheng W-W. Palliative chemotherapy near the end of life in oncology patients. American Journal of Hospice and Palliative Medicine®. 2018; 35: 1215-20.
- Peppercorn JM, Smith TJ, Helft PR, DeBono DJ, Berry SR, Wollins DS, et al. American society of clinical oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol. 2011; 29: 755-60.
- Chan W-l, Lam K-o, Siu W-k, Yuen K-k. Chemotherapy at end-of-life: an integration of oncology and palliative team. Supportive Care in Cancer. 2016; 24: 1421-7.
- Harrington SE, Smith TJ. The role of chemotherapy at the end of life:“when is enough, enough?”. Jama. 2008; 299: 2667-78.
- Organization WH. Strengthening of palliative care as a component of comprehensive care throughout the life course. Sixty-seventh World Health Assembly. 2014: 9-14.
- Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. Journal of the American Geriatrics Society. 2007; 55: 993-1000.
- Gans D, Hadler MW, Chen X, Wu S-H, Dimand R, Abramson JM, et al. Cost analysis and policy implications of a pediatric palliative care program. Journal of pain and symptom management. 2016; 52: 329-35.
- Lustbader D, Mudra M, Romano C, Lukoski E, Chang A, Mittelberger J, et al. The impact of a home-based palliative care program in an accountable care organization. Journal of palliative medicine. 2017; 20: 23-8.
- Kerr CW, Donohue KA, Tangeman JC, Serehali AM, Knodel SM, Grant PC, et al. Cost savings and enhanced hospice enrollment with a home-based palliative care program implemented as a hospice–private payer partnership. Journal of palliative medicine. 2014; 17: 1328-35.
- Wiencek C, Coyne P, editors. Palliative care delivery models. Seminars in oncology nursing. Elsevier. 2014.
- Kim SL, Tarn DM. Effect of primary care involvement on end-of- life care outcomes: a systematic review. Journal of the American Geriatrics Society. 2016; 64: 1968-74.
- Owens D, Eby K, Burson S, Green M, McGoodwin W, Isaac M. Primary Palliative Care Clinic Pilot Project demonstrates benefits of a nurse practitioner-directed clinic providing primary and palliative care. Journal of the American Academy of Nurse Practitioners. 2012; 24: 52-8.
- Baygi MZ, Seyedin H, Salehi M, Sirizi MJ. Structural and contextual dimensions of Iranian primary health care system at local level. Iranian Red Crescent Medical Journal. 2015; 17.
- Rassouli M, Shirinabadi Farahani A, Khanali Mojen L. Palliative care perspectives and practices in the Islamic republic of Iran, and their implication on patients’ quality of life. Palliative Care: Perspectives, Practices and Impact on Quality of Life New York: Publisher In Press, Nova Scientific. 2017.
- De Roo ML, Francke AL, Van den Block L, Donker GA, Alonso JEL, Miccinesi G, et al. Hospitalizations of cancer patients in the last month of life: quality indicator scores reveal large variation between four European countries in a mortality follow-back study. BMC palliative care. 2014; 13: 1-8.
- Cheung MC, Croxford R, Earle CC, Singh S. Days spent at home in the last 6 months of life: a quality indicator of end of life care in patients with hematologic malignancies. Leukemia & Lymphoma. 2020; 61: 146-55.
- van Baal K, Schrader S, Schneider N, Wiese B, Stahmeyer JT, Eberhard S, et al. Quality indicators for the evaluation of end-of-life care in Germany–a retrospective cross-sectional analysis of statutory health insurance data. BMC Palliative care. 2020; 19: 1-10.
- Wright AA, Zhang B, Keating NL, Weeks JC, Prigerson HG. Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: prospective cohort study. Bmj. 2014; 348: g1219.
- Prigerson HG, Bao Y, Shah MA, Paulk ME, LeBlanc TW, Schneider BJ, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA oncology. 2015; 1: 778-84.
- Karver SB, Berger J. Advanced care planning–empowering patients for a peaceful death. Asian Pac J Cancer Prev. 2010; 11: 23-5.
- De Schreye R, Houttekier D, Deliens L, Cohen J. Developing indicators of appropriate and inappropriate end-of-life care in people with Alzheimer’s disease, cancer or chronic obstructive pulmonary disease for population-level administrative databases: a RAND/UCLA appropriateness study. Palliative medicine. 2017; 31: 932-45.
- Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? Journal of clinical oncology. 2008; 26: 3860.
- Wright AA, Zhang B, Keating NL, Weeks JC, Prigerson HG. Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: prospective cohort study. BMJ: British Medical Journal. 2014; 348: g1219.
- Keam B, Oh D-Y, Lee S-H, Kim D-W, Kim MR, Im S-A, et al. Aggressiveness of cancer-care near the end-of-life in Korea. Japanese journal of clinical oncology. 2008; 38: 381-6.
- Akdeniz M, Yardimci B, Kavukcu E. Ethical considerations at the end-of-life care. SAGE open medicine. 2021; 9: 20503121211000918.