
Special Article: Prostate Cancer
Ann Hematol Onco. 2024; 11(3): 1454.
Is It Metastasis or Sweet’s Syndrome? A Diagnostic Dilemma and Review of Literature
Prateek Mehra¹*; Daniel Tesolin¹; Ana-Alicia Beltran-Bless²; Julia Malone¹; Christina Maria Bruna Canil²; Shawn Malone¹
¹Division of Radiation Oncology, The Ottawa Hospital Cancer Centre, Ottawa Hospital Regional Cancer Program, Ottawa, Canada
²Division of Medical Oncology, The Ottawa Hospital Cancer Centre, Ottawa Hospital Regional Cancer Program, Ottawa, Canada
*Corresponding author: Prateek Mehra, MD The Ottawa Hospital – General Campus, 501 Smyth Road, Ottawa, ON, K1H 8L6, Canada. Email: pmehra@toh.ca
Received: June 04, 2024 Accepted: July 01, 2024 Published: July 08, 2024
Abstract
Background: Sweet’s syndrome is a neutrophilic dermatosis, commonly associated with hematologic malignancies, infections, inflammatory bowel disease, medications, and pregnancy. It is rarely seen with solid tumors.
Case Presentation - We discuss a case here of a 60-year-old male with metastatic carcinoma prostate who presented with the classic Sweet’s syndrome lesions. The lesions did not respond to topical steroids, however they did resolve after starting the patient on androgen deprivation therapy, darolutamide and docetaxel chemotherapy. This case is a great display of a possible treatment option of Sweet’s syndrome associated with a solid tumor; a condition which is usually treated with corticosteroids or other immunosuppressants.
Conclusion - Malignancy should be kept in mind as an underlying cause while diagnosing Sweet’s syndrome and it should be remembered that this is a condition that can resolve with the treatment of the underlying malignancy.
Keywords: Sweet’s syndrome; Carcinoma prostate; Androgen deprivation therapy; Triplet therapy; Neutrophilic dermatosis; Paraneoplastic syndrome
Background
Sweet’s Syndrome is a disorder classified as a neutrophilic dermatosis characterized by the accumulation of neutrophils in the skin. Patients present with a sudden onset of fever and rash consisting of multiple tender erythematous nodules or lesions on the upper extremities, face, and neck [1]. It most commonly presents in middle aged women [2]. It is most commonly idiopathic in etiology but around 10-15% of the time be part of a paraneoplastic syndrome related to malignancy [3]. In case of paraneoplastic disease, it is associated with a hematologic malignancy about 85 % of the time. Rarely, Sweet’s Syndrome can be related to solid tumors and even more rarely, adenocarcinoma of the prostate [3]. Most commonly, it is treated with systemic corticosteroids [4]. We discuss a case here of a patient with metastatic carcinoma prostate who presented with Sweet’s syndrome.
Case Presentation
A 60-year-old male presented to his Family Physician with generalized fatigue, progressive obstructive urinary symptoms, bone pain and rapidly growing skin nodules. On physical examination, the digital rectal exam revealed a large, fixed prostate tumor mass. Prostate Specific Antigen (PSA) was elevated at 3240 ng/mL. His past medical history was significant for celiac disease and ulcerative colitis; however, his colitis had been in remission for more than a decade and he was not on any active medications. He was urgently referred to the Cancer Assessment Centre.
Staging investigations were arranged including a bone scan and Computed Tomography (CT) of his chest, abdomen, and pelvis. Imaging revealed widespread sclerotic metastatic disease throughout the skeleton, including the spine, pelvis, proximal femurs, ribs, sternum, scapulae, and left clavicle (Figure 1). Additionally, there were bulky hilar, mediastinal, and retroperitoneal nodes. Metastatic lymph nodes in the pretracheal, subaortic, subcarinal and bilateral hilar regions were up to 30mm in size, periaortic lymph nodes up to 23mm in size and right external iliac lymph nodes 57x23mm in size.
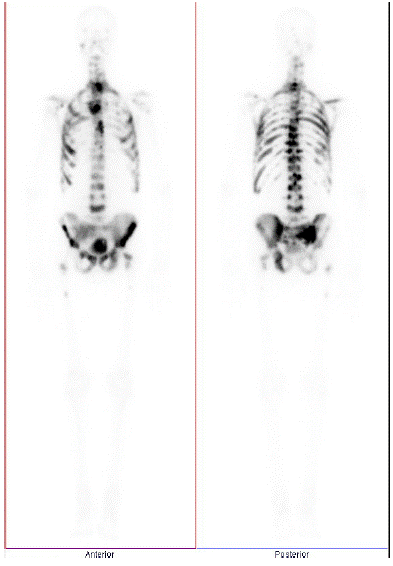
Figure 1: Technetium Bone scan during initial staging workup, before start of any systemic therapy.
The examination by his urologist revealed a bilateral tumour in his prostate that was clinically fixed to the pelvic side wall. The patient was started on bicalutamide and LHRH agonist therapy. The patient initially refused a prostate biopsy. He was referred to medical and radiation oncology for urgent consultations. The patient then agreed to a TRUS-guided prostate biopsy which revealed a Gleason score 4+5 adenocarcinoma in 12/12 cores. There was evidence of perineural invasion and extra-prostatic extension. There was no evidence of neuroendocrine differentiation of his cancer.
On assessment at the cancer clinic, the patient complained of rapidly progressive tender skin nodules on his face and dorsum of his hands. The skin nodules were his most distressing symptom. On enquiry, there was no history of prior dermatologic conditions. Examination revealed violaceous nodular skin lesions (Figure 2A & 2B) on his left thumb, proximal interphalangeal joint and right index metacarpophalangeal joint. There was superficial erosion and bleeding from the lesions. In addition, there was a 5mm erythematous non-ulcerated tender papule on his left cheek.
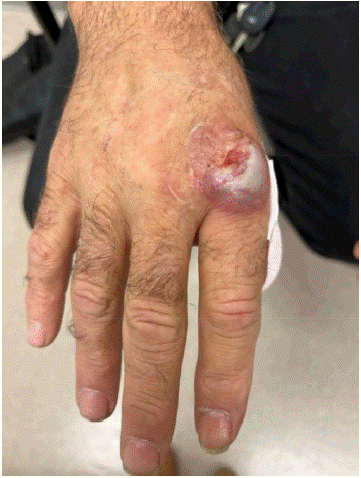
Figure 2A: Sweet’s syndrome lesion over right index metacarpophalangeal joint before start of any systemic therapy.
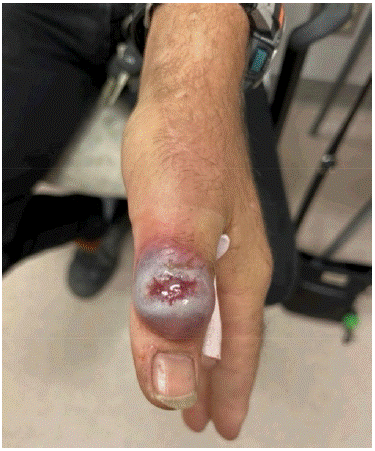
Figure 2B: Sweet’s syndrome lesion over left thumb before start of any systemic or topical therapy.
He was referred to dermatology who performed punch biopsies of both lesions on his hands. Clinically, the differential diagnoses included bullous dermatosis, pyoderma gangrenosum, lymphoma cutis, leukemia cutis and subcutaneous metastasis. Pathology revealed neutrophilic dermatosis, consistent with Sweet’s syndrome. The skin biopsy did not reveal any infectious pathogen or malignancy. Bloodwork was done to rule out a hematologic malignancy or flare up of his ulcerative colitis. The patient had an elevated ESR of 46 mm/hr and slightly elevated CRP of 12.7 mg/L. He had a mild anemia (hemoglobin of 112g/L) and otherwise normal complete blood count and normal serum protein electrophoresis.
Dermatology recommended topical steroids (clobetasol 0.05% ointment) and treatment of the underlying malignancy. The patient discontinued topical steroids after the first 2 weeks due to lack of response. The lesions resolved over the coming months with management of his underlying prostate cancer (Figure 3).
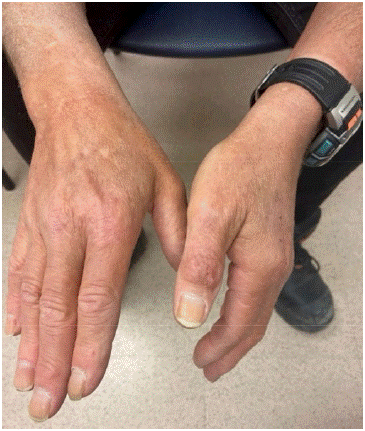
Figure 3: Resolution of Sweet’s syndrome lesions one month after completion of chemotherapy.
Medical oncology recommended triplet therapy with docetaxel chemotherapy and darolutamide in combination with his LHRH agonist therapy. Darolutamide was initiated about 2 weeks after prostate biopsy. Docetaxel was added 10 weeks after initiation of LHRH therapy. His PSA trended down to 280 ng/mL after two months of LHRH therapy. After seven months of treatment, his PSA was 17.5 ng/mL and restaging scans revealed significant decrease in the extent of lymphadenopathy and significant improvement in bone metastases (Figure 4). Follow up with dermatology 6 months after starting treatment of his prostate cancer revealed that his skin lesions had completely resolved.
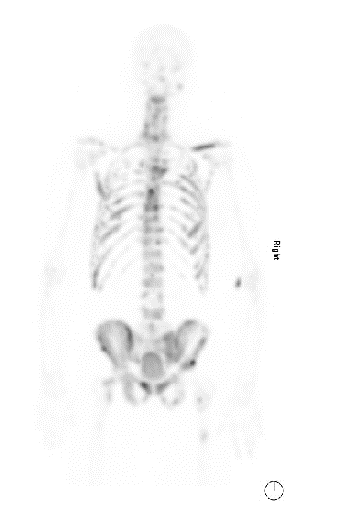
Figure 4: Improvement in bone metastases with triplet therapy as seen on restaging one month after completion of chemotherapy.
Patient tolerated triplet therapy well, with no need for dose reduction in the chemotherapy or the darolutamide. He completed 6 cycles of docetaxel and remains on ADT with darolutamide. CT scan for response assessment after chemotherapy revealed persistent metastatic disease with diffuse bone metastases but significant decrease in nodal metastases. PSA decreased from 2824 ug/L to 7.53 ug/L. Dermatology continues to follow up with the patient for consideration of immunosuppressive medications or Intralesional Kenalog injections (ILK) if recurrence of the lesions occurs.
Discussion
Sweet’s syndrome, while having been investigated heavily, has many clinical facets that remain unknown. The exact etiology of Sweet’s Syndrome remains unclear, with many possible theories for its cause and pathophysiology. There are 5 major possible causes of Sweet’s syndrome – cancer, infections, inflammatory bowel disease, medications, and pregnancy [5]. It is likely that the cause is multifactorial. Since many patients experience fever and show peripheral leukocytosis, an infectious process is suspected [6]. This is reinforced by the fact that tonsillitis or upper respiratory tract infections often precede the skin lesions by 1-3 weeks. Hence, a bacterial infection could be a reason behind the syndrome. Another possible cause is elevated granulocyte-colony stimulating factor [6]. Some cases of Sweet’s Syndrome have been associated with administration of G-CSF [7].
The biopsy of skin lesions in Sweet’s Syndrome reveals diffuse inflammatory infiltrates of neutrophils in the dermis, the subcutaneous fat or in both. A systemic examination may reveal elevated white blood cell count, elevated erythrocyte sedimentation rate and C-reactive protein. Sweet’s Syndrome can lead to a wide variety of clinical manifestations outside the skin. Extracutaneous Sweet’s Syndrome can cause a number of clinical symptoms depending on the location of the inflammatory infiltrates, including encephalitis, arthritis, hepatitis and stomatitis.
Sweet’s syndrome has a long list of possible differential diagnoses because many conditions can mimic one or multiple clinical manifestations associated with it. Cutaneous conditions that present similarly to Sweet’s syndrome include drug eruptions, acral erythema and rosacea fulminans [8]. Many infectious conditions present similarly, such as sepsis, cellulitis, herpes simplex infection, syphilis, panniculitis, and tuberculosis. Reactive erythema such as erythema multiforme, and erythema nodosum can present with similar symptoms. There are also many conditions that mimic Sweet’s syndrome histologically. These range from granuloma faciale and halogenoderma to leukemia cutis and pyoderma gangrenosum.
Most frequent symptom at presentation of Sweet’s syndrome is fever which is associated with skin lesions. The fever can precede the skin lesions by several days. Other symptoms which can be present include general malaise, headache, arthralgias, and myalgias. The skin lesions are commonly tender papules or nodules that are red in color. Skin lesions are mostly present over the face, neck, and upper extremities. The lesions can coalesce to form plaques over time. Resolution after treatment tends to be without scarring. There are many possible extracutaneous manifestations of Sweet’s syndrome as well. Cases have been reported with arthritis, encephalitis, blepharitis, glomerulonephritis, aortic stenosis, pleural effusion, oral ulceration, and myositis.
There are pathologic criteria for Sweet’s syndrome which include a diffuse infiltrate of mature neutrophils, local edema, leukocytoclasia and dilated small blood vessels. The overlying epidermis does not show much pathological change. Although the neutrophilic infiltrate is mostly found in the dermis, it can sometimes be present in the epidermis or in the underlying adipose. Extracutaneous manifestations have sterile neutrophilic inflammation of the organ which is affected. While neutrophils are most commonly associated with Sweet’s syndrome, eosinophils and lymphocytes have also been found in these inflammatory foci.
Sweet’s syndrome has a number of management options including treating the lesions themselves or treating the underlying responsible condition. Inn cases of paraneoplastic Sweet’s syndrome, resolution of the syndrome can occur with treatment of the underlying malignancy. Tender skin nodules are generally managed with topical or intralesional corticosteroids. Systemic corticosteroids, e.g., prednisone, form the gold standard of treatment for Sweet’s syndrome especially when associated with other systemic symptoms. Other first line systemic agents include potassium iodide and colchicine. Second-line systemic agents are indomethacin, clofazimine, cyclosporin and dapsone. In a minority of patients, the lesions resolve spontaneously. Drug-related Sweet’s syndrome usually resolves with the discontinuation of the offending medication. In malignancy-associated Sweet’s syndrome, while the skin condition can resolve with the treatment of the underlying malignancy, there can be a resurgence with the relapse of the cancer.
There are very few reports of Sweet’s syndrome associated with prostatic adenocarcinoma in the literature [9-12]. The few that are reported have had some confounding factor such as starting a new medication, or concurrent hematological or other malignancy. In almost all cases, the Sweet’s syndrome is treated effectively with corticosteroids. There is one older case report of a patient with metastatic prostate cancer developing Sweet’s syndrome that resolved and recurred without corticosteroids; however, it is unclear from the report if he was receiving any systemic treatment for his malignancy [11].
Our report is unique compared to those described in the literature as it describes a case of Sweet’s syndrome in the context of de-novo metastatic prostate adenocarcinoma. The patient had no other concurrent malignancy. The Sweet’s syndrome was felt to be a paraneoplastic syndrome and responded solely to effective therapy of his primary prostate cancer.
It should be noted that this patient also has a history of celiac disease and ulcerative colitis, possibly making him more predisposed to an episode of Sweet’s syndrome given his baseline dysregulated immune system. In our case, the patient did not present with any gastrointestinal symptoms, and his colitis had been in remission long term without medication. In addition, the Sweet’s syndrome resolved completely without any systemic corticosteroids. With initiation of androgen deprivation therapy followed by triplet therapy, the patient’s symptoms resolved with time, suggesting a paraneoplastic etiology in this case.
This case report highlights the importance of considering malignancy when making the diagnosis of Sweet’s syndrome. It also highlights that Sweet’s syndrome can resolve with treatment of the underlying cancer.
Author Statements
Declarations
Clearance from the Research Ethics Board - The Ottawa Hospital and patient consent for publication have been obtained prior to submission of manuscript.
The authors declare that they have no competing interests.
No sources of funding have been necessary for this case report.
Author’s Contributions
Prateek Mehra - Conception of work, interpretation of data, drafted the manuscript; Daniel Tesolin - Conception of work, acquisition and interpretation of data, drafted the manuscript; Ana-Alicia Beltran-Bless - Acquisition and interpretation of data; Julia Malone - Analysis of data, drafted the manuscript; Christina Maria Bruna Canil - Acquisition and interpretation of data; Shawn Malone - Conception of work, acquisition and interpretation of data, drafted the manuscript. All authors have approved the submitted version and have agreed both to be personally accountable for their own contributions and have ensured that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
References
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet Syndrome: A review and update. Actas Dermo-Sifiliográficas (English Edition). 2016; 107: 369-378.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003; 42: 761-778.
- Paydas S. Sweet’s syndrome: a revisit for hematologists and oncologists. Crit Rev Oncol Hematol. 2013; 86: 85-95.
- Cohen PR, Kurzrock R. Sweet’s syndrome: a review of current treatment options. Am J Clin Dermatol. 2002; 3: 117-131.
- Cohen PR. Sweet’s syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007; 2: 34.
- Reuss-Borst MA, Pawelec G, Saal JG, Horny HP, Müller CA, Waller HD. Sweet’s syndrome associated with myelodysplasia: possible role of cytokines in the pathogenesis of the disease. Br J Haematol. 1993; 84: 356-8.
- Kaya Z, Belen FB, Akyürek N. Granulocyte Colony Stimulating Factor Induced Sweet’s Syndrome Following Autologous Transplantation in a Child with Relapsed Acute Myeloblastic Leukemia. Indian J Hematol Blood Transfus. 2014; 30: 376-8.
- Cohen PR, Kurzrock R. Sweet’s syndrome and cancer. Clin Dermatol. 1993; 11: 149-57.
- Barnadas MA, Sitjàs D, Brunet S, Puig J, de Moragas JM. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) associated with prostate adenocarcinoma and a myelodysplastic syndrome. Int J Dermatol. 1992; 31: 647-648.
- Hussein K, Nanda A, Al-Sabah H, Alsaleh QA. Sweet’s syndrome (acute febrile neutrophilic dermatosis) associated with adenocarcinoma of prostate and transitional cell carcinoma of urinary bladder. J Eur Acad Dermatol Venereol. 2005; 19: 597-599.
- Dyall-Smith D, Billson V. Sweet’s syndrome associated with adenocarcinoma of the prostate. Australas J Dermatol. 1988; 29: 25-27.
- Glendenning J, Khoo V. Sweet’s syndrome in prostate cancer. Prostate Cancer Prostatic Dis. 2008; 11: 397-398.