
Case Report
Ann Hematol Onco. 2024; 11(4): 1460.
Visceral Leishmaniasis Mimicking Myelodysplastic Syndrome
Anwarul Islam, MD, PhD, FRCPath, FACP*
Clinical Associate Professor of Medicine, State University of New York at Buffalo, Attending Physician, Division of Hematology/Oncology, Department of Medicine, Buffalo General Medical Center, USA
*Corresponding author: Anwarul Islam, MD, PhD, FRCPath, FACP Clinical Associate Professor of Medicine, State University of New York at Buffalo, Attending Physician, Internal Medicine-Hematology/Oncology, Buffalo General Medical Center, Buffalo, New York 14203, USA Email: aislam@buffalo.edu
Received: July 27, 2024 Accepted: August 08, 2024 Published: August 15, 2024
Abstract
We present the case of a 71-years-old white female who presented to the emergency department with a two-week history of fever of unknown origin, mild anemia and marked thrombocytopenia. The patient was admitted to the hospital and was treated with a high dose broad spectrum antibiotic. The patient also gave a history of returning from an African country where the disease is endemic about three months prior to her presentation at ER. Because of her hematologic findings (anemia and marked thrombocytopenia) she underwent a bone marrow examination which revealed the presence of Leishman Donovan bodies. Furthermore, the bone marrow aspirate smear revealed marked dysmyelopoietic changes involving all three hematopoietic cell lines akin to features commonly seen in patients with Myelodysplastic Syndrome (MDS).
Keywords: Visceral leishmaniasis; Kala-azar; Myelodysplastic syndrome; Leishman Donovan bodies; Bone marrow aspiration; Bone marrow biopsy
Introduction
Visceral Leishmaniasis (VL) is a disseminated protozoal infection, transmitted by sandfly bite, in which macrophages of the liver, spleen and bone marrow are parasitized and become sites of their intracellular replication [1]. There are an estimated 500,000 new global cases per year [2]. Although 90% of the new cases occur in just five countries (India, Bangladesh, Brazil, Nepal and Sudan) [3], they are also seen in Europe, African, Asian, and Latin American countries. As travel patterns shift it is a disease that is being frequently introduced into developed countries.
In developed countries VL is suspected when an individual who has been in an endemic area develops, fever, splenomegaly, anemia, neutropenia or thrombocytopenia either singly or in combination [4]. Coagulation abnormalities including disseminated intravascular coagulation may also be observed [5,6]. Bone marrow aspiration and biopsy is a useful method of diagnosing or confirming the diagnosis [7,8].
Case Report
The patient is a 71 year-old-white female with a past medical history significant for anxiety, gastroesophageal reflux disease, hyperlipidemia, hypertension, diabetes mellitus type II who presented at the hospital emergency department with complaints of fever, body aches, arthralgia, frontal headache, and dizziness intermittently over the last two weeks. She reported a fever as high as 104°F. Of note, she returned from Africa about four months before the presentation at the ER when she felt ill for two weeks with similar symptoms but tested positive for COVID-19. While in the emergency department, the patient remained hemodynamically stable. She complained of chills and her temperature was 99.8°F. The laboratory work-up at the ER revealed WBC 1.7 x109/L, hemoglobin 10.3 g/dL, and platelet 273 x109/L. She was COVID, and Epstein-Barr virus negative, troponin negative x 2. Urine analysis was unremarkable and creatinine was slightly raised at 1.79 mg/dL. She was admitted to the hospital for further evaluation of neutropenic fever of unknown origin. The infectious disease specialist was consulted who recommended antibiotic therapy. Multiple bacteriology and virology tests were performed including syphilis, TB, HIV, CMV, EVB, Flu A, and B which were all negative. The heterophile antibody test (mono spot) was negative. The patient’s flow cytometry revealed markedly reduced granulocyte gate with circulating CD 34-positive blasts accounting for approximately 1% of total events. There also was basophilia, eosinophilia, and monocytosis present. The flow cytometry findings suggested an involvement with a hematopoietic neoplasm. A bone marrow aspiration and biopsy with cytogenetics and molecular studies were recommended for further diagnostic evaluation. It was performed and the smears of bone marrow aspirate revealed myelodysplastic changes in erythroid (Figure 1), granuloid (Figure 2), and megakaryocytic cells (Figure 3). Plasma cells, eosinophilic granulocytes, and macrophages were prominent. Most importantly numerous amastigotes (the tissue form of the Leishmania parasite) were seen within and external (paracellular) to the mononuclear phagocytes (Figure 4) and of note cytogenetic studies revealed loss of part of the long arm (q arm) of chromosome 5, also known as 5q minus (5q-). Her clinical course continued to improve, her fever resolved, her hemoglobin and WBC count returned to normal and she was discharged to her home. At present for about three months following her discharge from the hospital she has been symptom-free with a normal complete metabolic profile and hematologic indices. Although serology tests for leishmania immunoglobulins, confirmatory culture testing, polymerase chain reaction, and DNA sequencing analysis were negative, the presence of Leishmania Donovan bodies (amastigotes) (Figure 4) in the bone marrow, clinical features (intermittent fever, arthralgia), splenomegaly and a recent trip to Africa was highly suggestive that the patient did have leishmaniasis. Furthermore, unlike MDS her blood indices returned to normal without any further treatment.
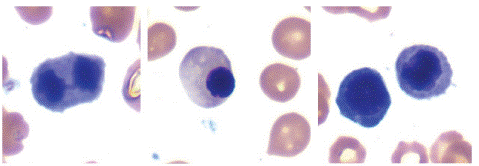
Figure 1: Bone marrow aspirate smear showing dyserythropoietic changes. Note internuclear bridging, asynchrony in nuclear cytoplasmic maturation, irregular nuclear and cytoplasmic outlines.
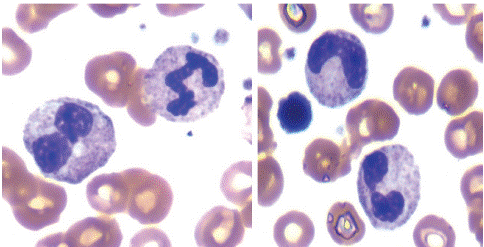
Figure 2: Bone marrow aspirate smear showing dysgranulopoiesis. Note Pelger forms, dumbbell forms and bizarre nucleus.
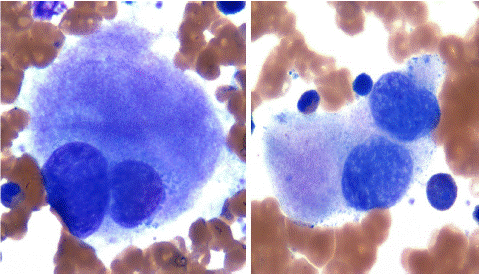
Figure 3: Bone marrow aspirate smear showing dysmegakaryopoiesis. Note bi and multinucleated megakaryocytes.
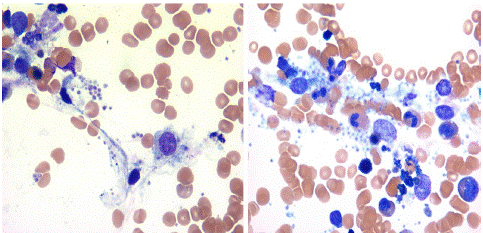
Figure 4: Bone marrow aspirate smear showing hystiocytes with intra and extra cellular Leishman Donovan bodies.
Discussion
Visceral Leishmaniasis or kala-azar (Hindi for “black fever”) is a well-recognized disseminated zoonotic viscerotropic protozoal infection mainly affecting malnourished and underprivileged children in the endemic areas of the world [7]. Visceral leishmaniasis was first described in 1903 by physicians Leishman and Donovan who demonstrated parasites in stained smears from spleens of patients suffering from malaria-like illnesses. The incubation period of visceral leishmaniasis is usually 2-4 months but it can be as long as 10 years or more. Recently, this disease has been seen in acquired immunodeficiency syndrome patients and in travelers in the endemic areas [7]. Visceral leishmaniasis is a systemic disease that can cause fever, weight loss, anemia, and enlargement of the spleen, liver, and lymph nodes. Anemia and neutropenia are always present. Reduction of platelet counts usually completes the picture of progressive pancytopenia which is typical of visceral leishmaniasis [5]. Visceral leishmaniasis is a challenging disease to treat and if left untreated, can be fatal in more than 90% of cases. There are no vaccines or drugs to prevent infection, but travelers can protect themselves from sandfly bites.
VL has traditionally been diagnosed by finding the parasites in tissue fluids or in histological sections, especially bone marrow, lymph nodes and spleen [8]. Splenic aspirate is reputed to be the material most likely to reveal the parasites but they are difficult to perform, and not free of complications and morbity. Lymph node and bone marrow aspiration are easy to perform, usually free of complications and morbidity and is believed to be equal in sensitivity [8].
As stated earlier visceral leishmaniasis produces a variety of hematologic abnormalities, such as anemia, leukopenia, thrombocytopenia and pancytopenia [9]. The bone marrow, spleen and lever are common sites of infection, but other organs such as the lung, kidney and gastrointestinal tract can be involved [10].
Bone marrow aspiration in this condition shows macrophages/reticular cells loaded with Leishman Donovan bodies and a polymorphic population of plasma cells, lymphocytes, macrophages, reticular cells and immunoblasts. Myelodysplastic changes involving one or more hematopoietic cell lines in visceral leishmaniasis has been reported [7,11] but its etiology and significance remains unclear. Our patient showed extensive myelodysplastic changes in the bone marrow and if the patient did not give a history of recent visit to one of the endemic areas for visceral leishmaniasis in Africa in the background of features commonly associated with visceral leishmaniasis (stated earlier) particularly, fever, anemia, thrombocytopenia, arthralgia, splenomegaly and if we did not carefully looked for LD bodies in the bone marrow aspirate smears of this patient, her diagnosis of visceral leishmaniasis might have been missed and she might have been misdiagnosed as suffering from MDS. Hematologic changes such anemia, leucopenia and thrombocytopenia either singly or in combination are common in visceral leishmaniasis and we believe these changes reflect an in ineffective hematopoiesis due to parasitic infection of the bone marrow. Splenomegaly that results in increased pooling of blood cells within the spleen, an increase in plasma volume (hemodilution), may also be contributing.
It is unclear though why in the present case the tests for visceral leishmaniasis were negative. We are not sure if broad-spectrum antibiotic therapy has had something to do with this. In addition, her 5q minus in the background of the MDS features remains unclear. It is known however, that nearly 90% of the so-called MDS patients do not show this chromosomal abnormality. Furthermore, dyserythropoiesis in patients with visceral leishmaniasis [7] and leishmaniasis masquerading as MDS has also been reported [11]. This case thus illustrates the diagnostic pitfalls for myelodysplastic syndromes. Finally, although molecular and other methods of diagnosis of visceral leishmaniasis are more reliable [12] for the detection of organisms, negative results also do not exclude the possibility of the disease, as shown in our case.
References
- Henry W. Murray H W. Clinical and Experimental Advances in Treatment of Visceral Leishmaniasis. Antimicrob Agents Chemother. 2001; 45: 2185–2197.
- World Health Organization, Leishmaniasis Disease Burden.
- Moore EM, Lockwood DN. Treatment of Visceral Leishmaniasis. J Glob Infect Dis. 2010; 2: 151-158.
- Chulay JD, Bryceson ADM. Quantitation of amastigotes of leismania Donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am J Trop Med Hyg. 1983; 32: 475–479.
- Al-Jurayyan NAM, Al-Nasser MN, Al-Fawaz IM, Al Ayed IH, Al Herbish AS, Al-Mazrou AM, et al. The Haematological Manifestations of Visceral Leishmaniasis in Infancy and Childhood. Journal of Tropical Pediatrics. 1995; 41: 143-148.
- Li VS, Fischer A, Musumeci S. Hematological and Serological aspects of Nediterranean Kala-Azar in infancy and childhood. Acta Tropica. 1980; 37: 351-365.
- Sheikha A. Dyserythropoiesis in 105 Patients with Visceral Leishmaniais. Laboratory hematology. 2004; 10: 206-11.
- Siddig M, Ghalib H, David C. Visecral lieshmaniasis in the Sudan: comparative parasitological methods of diagnosis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988; 82: 66-68.
- Daneshbod Y, Deghani SJ, Daneshbod K. Bone Marrow Aspireation Findings in Kala-Azar. Acta Cytologica. 2010; 54: 12-24.
- Daneshbod K. Visceral leishmaniasis (kala-azar) in Iran: A pathologic and electron microscopic studies. Am J Clin Pathol. 1972; 57: 156-166.
- Kopterides P, Halikias S, Tsavaris N. Visceral Leishmaniasis Masquerading as Myelodysplasia. Am J Hem. 2003; 74: 198-199.
- Clement PW, Li KD. Peripheral blood and bone marrow involvement by visceral leishmaniasis. Blood. 2017; 130: 692.