
Case Report
Ann Hematol Oncol. 2025; 12(3): 1483.
A Case of Hairy Cell Leukemia in a Patient with Sarcoidosis: Diagnostic and Treatment Challenges
Zatsepina A¹*, Winn SP¹, Sayeed S¹, Giverts I¹, Shyam T¹, AlShihabi A¹, Chaudhry MA¹, Podberezin M² and Peeke SZ³
1Department of Internal Medicine, Maimonides Medical Center, USA
2Department of Hematopathology, Maimonides Medical Center, USA
3Department of Hematology and Oncology, Montefiore Medical Center, USA
*Corresponding author: Zatsepina A, Department of Internal Medicine, Maimonides Medical Center, 4802 Tenth Avenue, Brooklyn, NY 11219, USA Tel: +17182836000; Email: zatsepina.alina@gmail.com
Received: June 15, 2025 Accepted: July 15, 2025 Published: July 18, 2025
Abstract
Hairy cell leukemia (HCL) is a rare B-cell malignancy that can present diagnostic challenges when coexisting with other conditions. Sarcoidosis, a multisystem granulomatous disease, shares several overlapping clinical features with HCL, complicating timely diagnosis and treatment initiation.
We present a case of a 44-year-old male with a history of sarcoidosis and mild normocytic anemia who developed worsening cytopenias and splenomegaly after a severe COVID-19 infection. Initial hematologic abnormalities were attributed to sarcoidosis-related inflammation and post-viral bone marrow suppression. However, persistent anemia and thrombocytopenia led to further evaluation, revealing atypical lymphocytes with “hairy” projections. Bone marrow biopsy confirmed HCL with the BRAF V600E mutation. First-line treatment resulted in partial remission, but residual disease persisted. The patient relapsed and was successfully treated with targeted therapy, achieving sustained complete remission for over a year.
The co-occurrence of HCL and sarcoidosis presents a unique diagnostic challenge. This case highlights the diagnostic complexity of HCL in the setting of pre-existing sarcoidosis and recent severe infection. Given their overlapping clinical manifestations, distinguishing between the two conditions is crucial to prevent delays in appropriate treatment.
Keywords: Hairy cell leukemia; Sarcoidosis; Pancytopenia; Targeted therapy
Introduction
Hairy cell leukemia (HCL) is a rare B-cell malignancy that represents 2% of all leukemia cases. Approximately 1,100 new HCL diagnoses are made annually in the U.S. [1]. It is four to five times more common in men than in women and is defined by the presence of the BRAF V600E mutation in greater than 97% of cases [2].
Characterized by pancytopenia, splenomegaly, and a high susceptibility to infections, HCL presents a unique diagnostic challenge, particularly when it coexists with other multisystemic diseases.
Sarcoidosis is characterized by the formation of granulomas in multiple organs, with pulmonary involvement being the most common, affecting approximately 90% of cases. Extrapulmonary manifestations are also prevalent, with skin lesions, uveitis, liver and spleen involvement, lymphadenopathy, and arthritis occurring in 25- 50% of patients [3].
Case Presentation
A 44-year-old Hispanic male with a history of asthma, sarcoidosis, and chronic normocytic anemia was referred to hematology for worsening anemia and new-onset thrombocytopenia. His sarcoidosis was diagnosed in 2009 following symptoms of bronchitis and night sweats. Imaging revealed bilateral lung opacities, lymphadenopathy, and hepatic/splenic involvement. Bronchoscopic biopsy confirmed stage III sarcoidosis, and he was monitored without treatment.
The patient had no family history of hematologic or autoimmune disorders, was a nonsmoker, and used only as-needed albuterol. In 2019, blood work showed mild anemia (hemoglobin (Hb) 11.6 g/ dL) and leukopenia, attributed to anemia of chronic disease in the setting of known sarcoidosis.
In 2020, he was hospitalized with severe COVID-19 requiring ICU care and intubation. He recovered without long-term complications.
In February 2021, the patient presented with worsening anemia (Hb 10.1 g/dL), new thrombocytopenia (platelets 66 K/μL), and splenomegaly. He reported easy bruising but denied any constitutional symptoms. He had previously noted a sensation of firmness in his left upper quadrant. Physical examination revealed non-tender splenomegaly.
Peripheral smear revealed anisocytosis, spherocytes, and atypical lymphocytes. Initial evaluation suggested cytopenias were due to splenic sequestration, chronic inflammation, and potential post- COVID marrow suppression. However, labs progressively declined (Hb nadir 7.5 g/dL in June 2021), and further smears showed nucleated red blood cells (RBCs) and lymphoid cells with hairy projections (Figure 1).
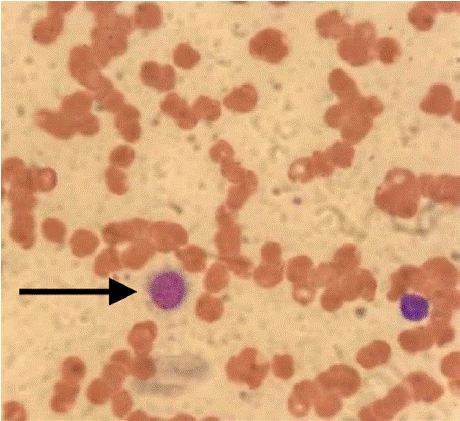
Figure 1: Peripheral blood smear.
Mild anisocytosis, macrocytosis, polychromasia; occasional larger lymphoid
cells with hairy projections (arrow);
neutrophils without dysplastic features; and decreased platelets.
Representative image of a peripheral blood
smear stained with Wright-Giemsa stain. The smear was prepared from a
venous blood sample. Image captured
at 1000x total magnification under oil immersion. Scale bar = 10 μm.
A trial of prednisone 20 mg daily was initiated due to worsening cytopenias and splenomegaly in the setting of known sarcoidosis. This led to transient absolute neutrophil count (ANC) improvement.
Peripheral blood flow cytometry identified a lambda-restricted B-cell population expressing CD11c, CD25, CD103, and aberrant CD2, with findings subsequently confirmed by bone marrow biopsy and aspirate (Figure 2). Next-generation sequencing (NGS) detected the BRAF V600E mutation, consistent with classic hairy cell leukemia.
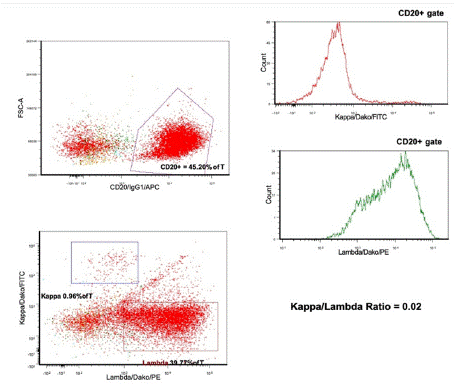
Figure 2: Bone marrow biopsy and aspirate results.
Flow cytometry reveals an abnormal Lambda restricted B cell population,
approximately 50% of total cells (K/L
ratio of 0.02), expressing CD19, CD20, CD11c, HLA-DR, CD25, CD103 and
CD2 - an immunophenotype
consistent with hairy cell leukemia.
FCS-A, forward scatter area; % of T, percent of total cells gated; APC,
allophycocyanin; FITC, fluorescein
isothiocyanate; PE, phycoerythrin.
Treatment with cladribine and concurrent weekly rituximab was initiated based on the trial by Chihara et al [4]. The regimen consisted of cladribine 0.15 mg/kg intravenously (IV) on days 1-5, along with eight weekly doses of rituximab, starting on day 1.
He tolerated cladribine well, with mild rituximab reactions and required transfusions for anemia. By September 2021, his labs and splenomegaly had improved.
Despite initial response, in January 2022, bone marrow biopsy revealed 11% residual HCL. He was observed further. By May 2022, minimal residual disease (<1%) was found. He received 4 weekly SQ rituximab/hyaluronidase doses in July 2022. He remained clinically well until November 2022, when he developed bacterial pneumonia and his hemoglobin declined.
In March 2023, relapse was confirmed with 8% HCL by flow cytometry (Figure 3). Given that the initial remission lasted less than 24 months, the next line of treatment was vemurafenib combined with SQ rituximab (two induction cycles + four consolidation doses) [5]. Treatment was interrupted briefly for pneumonia but resumed in May 2023 with mild toxicities (neutropenia, QTc prolongation, folliculitis). Pegfilgrastim was administered for neutropenia.
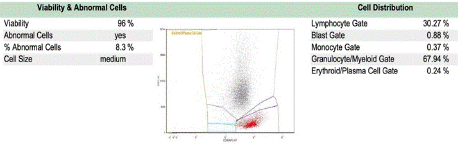
Figure 3: Peripheral blood flow cytometry showed recurrent/persistent
HCL.
A monoclonal lambda B-cell population (8.3% of total) expressing bright
intensity CD20 and CD11c is present.
This population co-expresses CD25 and CD103 and displays abnormally
bright CD19 and CD22 expression.
SSC-A, Side Scatter Area; APC-H7, Allophycocyanin-H7 (dye).
By July 2023, he completed therapy and achieved complete remission. September 2023 flow cytometry and BRAF testing were negative. A November 2023 bone marrow biopsy confirmed remission but showed a new del(20q) mutation. This cytogenetic abnormality was attributed to his prior chemotherapy and is planned for longterm monitoring [6]. Granulomas consistent with sarcoidosis were also noted.
As of June 2024, the patient remains in complete clinical and hematologic remission (Hb 13.7 g/dL, platelets 171 K/μL, white blood cells (WBC) 4.6 K/μL, ANC 3.7 K/μL). Trends in laboratory parameters throughout the course of illness and treatment are illustrated in Table 1.
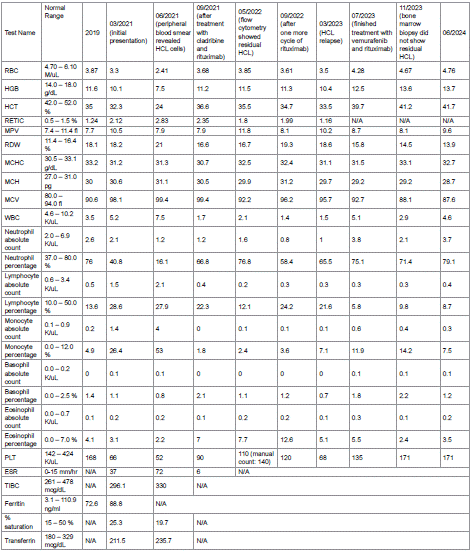
Table 1: Blood work results.
HCL, hairy cell leukemia; RBC, red blood cells; HGB, hemoglobin; HCT, hematocrit; RETIC, reticulocyte count; MPV, mean platelet volume; RDW, red cell
distribution width; MCHC, mean corpuscular hemoglobin concentration; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; WBC,
white blood cells; PLT, platelets; ESR, erythrocyte sedimentation rate; TIBC, total iron-binding capacity; BUN, blood urea nitrogen; ALT, alanine
aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; HIV 1/2 EIA, Human Immunodeficiency
Virus Type 1 and Type 2 Enzyme Immunoassay; PCR, Polymerase Chain Reaction; CMV, Cytomegalovirus; Ig, immunoglobulin; N/A, not analyzed.
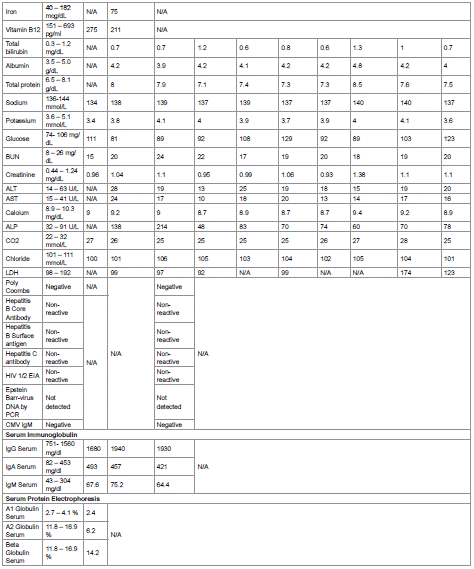
Table 1 of 1:
Discussion
Hairy cell leukemia is a rare type of lymphoid neoplasm characterized by the proliferation of mature B lymphocytes. Most cases are believed to arise from late-stage activated memory B cells that acquire a BRAF V600E mutation [2]. Patients usually present with frequent infections, pancytopenia and splenomegaly.
Purine analogs, such as cladribine and pentostatin, are the preferred first-line treatments for symptomatic HCL. Both achieve high complete remission (CR) rates, regardless of tumor burden [7]. Cladribine is often favored for its convenient administration. Cladribine alone achieves CR in 85-90% of cases, increasing to 90- 95% with an anti-CD20 monoclonal antibody rituximab.
Despite high remission rates, relapse remains a concern, and BRAF inhibitors (vemurafenib or dabrafenib) are an option for early relapses.
The coexistence of sarcoidosis and HCL is rare, with only a few cases reported. Our case is the only one in which HCL relapsed and was successfully treated with BRAF inhibitor and anti-CD20 monoclonal antibody, resulting in a complete hematological and clinical response. Additionally, a recent COVID-19 infection further complicated the diagnostic picture in our patient.
In the case report described by Karadurmus N. et al. [8], a patient had been diagnosed with sarcoidosis and massive splenomegaly and treated with corticosteroid therapy for 10-12 years. She was later diagnosed with HCL, and after receiving a single cycle of cladribine, she achieved remission. In a case described by Schiller G. et al. [9], a patient with sarcoidosis was treated with high-dose corticosteroids and developed asymptomatic leukopenia within four months, with morphological and immunophenotypic findings confirming HCL. The patient subsequently received cladribine, after which a follow-up TRAP stain on the peripheral smear was negative.
The patient described by Myers T. et al. in 1979 [10] presented with constitutional symptoms, bilateral anterior uveitis, knee arthritis, and erythema nodosum. She was diagnosed simultaneously with sarcoidosis and HCL. Following splenectomy (treatment of choice at the time), her WBC and platelet counts increased.
Although both sarcoidosis and HCL present with lymphadenopathy and splenomegaly, which can cause diagnostic confusion, the underlying pathophysiological mechanisms of these conditions are distinct. In sarcoidosis, these symptoms result from autoimmune granulomatous inflammation caused by an exaggerated T-helper cell response. In contrast, in HCL, the symptoms arise from the abnormal accumulation of clonal B cells. Reports of granulomatous-like reactions following the introduction of immunotherapies or targeted therapies further complicate the diagnosis of either sarcoidosis or malignancy [11].
Previous case reports suggest that defects in T helper cells caused by sarcoidosis may promote B lymphocyte proliferation and the development of lymphoproliferative disorders [10,12]. A possible explanation for this is the coordinated interaction between T cells and B cells, where the dysregulated activation of helper T cells leads to the activation of effector B cells. This occurs through direct cell-cell interactions, involving the CD40 ligand on activated T-helper cells binding to the CD40 protein on B cell surfaces. Furthermore, there are case reports highlighting the phenomenon of sarcoidosis-lymphoma association, revealing a complex relationship between sarcoidosis and cancer, especially non-Hodgkin lymphoma [11]. One retrospective exploratory case-control study conducted by Anderson et al. found that sarcoidosis is associated with an increased risk of subsequent HCL within 1 to 5 years of the initial diagnosis of sarcoidosis. This further supports the hypothesis of the sarcoidosis-lymphoma phenomenon, possibly due to the chronic activation of the immune system by sarcoidosis and the close interaction between T and B cells [13]. How an inflammatory state induced by severe COVID-19 infection could contribute to this process is unknown.
Another noteworthy point is that twenty-eight months after beginning cladribine treatment, the patient developed a new del(20q) mutation, which was confirmed through a bone marrow biopsy. Isolated del(20q) is not uncommon in patients who have undergone cytotoxic therapies, though its clinical significance in this context remains unclear. In a retrospective study by Yin, C. et al. [14], 92 patients were found to have acquired isolated del(20q) after receiving cytotoxic therapies for malignant neoplasms, two of whom had a diagnosis of HCL. The median time from prior therapy to del(20q) detection was 58 months (range: 5-213 months). With a median follow-up of 23 months (range: 1-183 months), 21 patients (23%) developed therapy-related myeloid neoplasms, while the remaining 71 patients (77%) did not. Thus, while the development of isolated del(20q) following cytotoxic therapy, as in this patient's case, does raise concerns about the potential for therapy-related myeloid neoplasms, it does not definitively predict such an outcome. The majority of patients in the study by Yin et al. did not progress to myeloid neoplasms, suggesting that while del(20q) may warrant careful monitoring, it is not necessarily an indicator of imminent transformation.
Conclusions
In conclusion, classic HCL is an uncommon hematologic malignancy with unique clinical and pathological features, driven by the BRAF V600E mutation.
The co-occurrence of HCL with sarcoidosis, as noted in several case reports, highlights the complexity of immune dysfunction in these conditions. Both disorders share overlapping symptoms, complicating diagnosis. The link between sarcoidosis and subsequent lymphoproliferative disorders like HCL may stem from chronic immune activation and dysregulated T and B cell interactions. These findings emphasize the importance of vigilance in patients with preexisting sarcoidosis, as early detection and management of HCL can significantly improve outcomes.
Finally, cytogenetic abnormalities, such as isolated del(20q), observed in some HCL cases following cytotoxic therapies, suggest the need for ongoing monitoring.
References
- Teras LR, DeSantis CE, Cerhan JR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016; 66: 443– 459.
- Falini B, Martelli MP, Tiacci E. BRAF V600E mutation in hairy cell leukemia: from bench to bedside. Blood. 2016; 128: 1918–1927.
- Sève P, Pacheco Y, Durupt F. Sarcoidosis: A clinical overview from symptoms to diagnosis. Cells. 2021; 10: 766.
- Chihara D, Arons E, Stetler-Stevenson M. Randomized Phase II study of firstline cladribine with concurrent or delayed rituximab in patients with hairy cell leukemia. J Clin Oncol. 2020; 38: 1527–1538.
- Tiacci E, De Carolis L, Simonetti E, et al. Vemurafenib plus rituximab in refractory or relapsed hairy-cell leukemia. N Engl J Med. 2021; 384: 1810– 1823.
- Courville EL, Singh C, Yohe S. Patients with a history of chemotherapy and isolated del(20q) with minimal myelodysplasia have an indolent course. Am J Clin Pathol. 2016; 145: 459–466.
- Else M, Dearden CE, Matutes E, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009; 145: 733–740.
- Karadurmus N, Erdem G, Basaran Y. A very rare case – hairy cell leukemia in a patient with sarcoidosis. Klin Onkol. 2015; 28: 215–217.
- Schiller G, Said J, Pal S. Hairy cell leukemia and sarcoidosis: a case report and review of the literature. Leukemia. 2003; 17: 2057–2059.
- Myers TJ, Granville NB, Witter BA. Hairy cell leukemia and sarcoid. Cancer. 1979; 43: 1777–1781.
- El Jammal T, Pavic M, Gerfaud-Valentin M. Sarcoidosis and cancer: a complex relationship. Front Med (Lausanne). 2020; 7: 594118.
- Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997; 336: 1224–1234.
- Anderson LA, Engels EA. Autoimmune conditions and hairy cell leukemia: an exploratory case-control study. J Hematol Oncol. 2010; 3: 35.
- Yin CC, Peng J, Li Y, et al. Clinical significance of newly emerged isolated del(20q) in patients following cytotoxic therapies. Mod Pathol. 2015; 28: 1014–1022.