
Research Article
Ann Hematol Oncol. 2025; 12(3): 1484.
Clinical Characteristics Analysis of 44 Cases of IgM Multiple Myeloma in China: A Multicenter, Retrospective, Observational Study
Meilan Chen*
Department of Hematology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
*Corresponding author: Meilan Chen, Department of Hematology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China Tel: 02013570585754; Email: chenmlan@mail.sysu.edu.cn
Received: July 22, 2025 Accepted: August 04, 2025 Published: August 07, 2025
Abstract
Objective: To analyze the clinical characteristics, treatment response, and prognosis of IgM-type multiple myeloma (MM) in China.
Methods: A multicenter, retrospective analysis was conducted on 44 newly diagnosed IgM- type MM patients from 16 MM centers in China between January 2015 to December 2024. Comparative analyses were performed with 618 non- IgM MM patients and 23 cases of Waldenström macroglobulinemia (WM).
Results: The mean age of IgM-type MM patients (63.68 years) was significantly older than that of non-IgM MM patients (58.27 years, p=0.003), but younger than WM patients (68.04 years, p=0.043). Compared to non-IgM MM, IgM-type MM patients exhibited higher serum albumin levels, lower β2- microglobulin levels, and a greater proportion of ISS stage I or R- ISS stage I-II disease (all p<0.05). In contrast to WM IgM-type MM patients demonstrated significantly elevated serum creatinine and calcium levels (p=0.008 and p=0.024, respectively). The t(11;14) translocation frequency in IgM-type MM (51.4%, 18/35) markedly exceeded that in non-IgM MM (12.6%, 76/601, p=0.000). WM patients showed significantly higher CD19+ expression by flow cytometry (83.3% vs 10.5%, p=0.006), while CCND1 positivity was more prevalent in IgMtype MM (29.6% vs 0%, p=0.040). No significant differences were observed in complete response (CR) rates (p=0.067) or =very good partial response (=VGPR) rates (p=0.067) between IgM and non-IgM MM following induction chemotherapy. Median progression-free survival (PFS) was 42.18 months for IgM-type MM versus 47.0 months for non-IgM MM (p=0.165), with median overall survival (OS) of 62.79 months and 63.54 months, respectively (p=0.736). Among IgM-type MM patients, those with concurrent t(11;14) translocation and CCND1 positivity demonstrated superior outcomes (median PFS: 101.0 months vs 71.0 months, p=0.378), though statistical significance was limited by sample size. Treatment regimens (PIs, PIs+Imids, or CD38 monoclonal antibodies) showed no significant survival differences (PFS p=0.577; OS p=0.186). Autologous stem cell transplantation (ASCT) in 5 IgM-type MM patients showed promising survival benefits (median PFS: 42.42 months vs NR, p=0.353; OS: 62.80 months vs NR, p=0.141).
Conclusion: IgM-type MM presents with older onset, earlier-stage disease by ISS or R-ISS criteria, and high t(11;14) incidence, yet demonstrates comparable prognosis to non-IgM- type MM. ASCT may significantly improve survival. Distinctive features including bone marrow morphology, flow cytometry profiles, cytogenetics, and MYD88 mutation status facilitate differentiation from WM. This study provides foundational evidence for understanding the clinicopathological and cytogenetic characteristics of Chinese IgM MM patients.
Keywords: IgM-type multiple myeloma; Clinical characteristics; t(11;14) translocation; Prognosis
Introduction
Multiple myeloma (MM) is a malignant hematologic disorder originating from plasma cells, characterized by heterogeneous immunoglobulin subtypes. IgM-type MM represents an exceptionally rare variant, accounting for only 0.5%-1.0% of all MM cases [1-8]. The limited incidence has resulted in insufficient characterization of its clinical features, particularly among Chinese populations. Diagnostic and therapeutic challenges persist due to its rarity and phenotypic overlap with Waldenström macroglobulinemia (WM). China currently lacks large-scale studies on IgM-type MM, while international data remain scarce and predominantly pre-2016. This multicenter retrospective study aims to delineate the clinical characteristics, cytogenetic profiles, and treatment responses of IgM-type MM in China, thereby establishing an evidence base for improved disease understanding.
Materials and Methods
Study Design
This multicenter retrospective observational study analyzed 44 IgM-type MM patients diagnosed between 2015-2024 across 16 Chinese MM centers. Comparator cohorts included 618 non-IgM MM and 23 WM patients during the same period, with comprehensive evaluation of clinical parameters, laboratory findings, and survival outcomes.
Eligibility Criteria
Inclusion criteria for IgM-type MM: The diagnosis met the criteria outlined in the 2014 IMWG guidelines for the diagnosis and treatment of multiple myeloma, with serum immunofixation electrophoresis (IFE) indicating the IgM type.
Inclusion criteria for Non-IgM MM: The diagnosis met the criteria outlined in the 2014 IMWG guidelines for the diagnosis and treatment of multiple myeloma, with serum immunofixation electrophoresis (IFE) indicating the non-IgM type.
Exclusion criteria (all groups): Monoclonal gammopathy of undetermined significance (MGUS), primary systemic light-chain amyloidosis, POEMS syndrome, and MGRS were ruled out.
Data Collection
Patient clinical data were collected, including age, sex, laboratory test results (such as hemoglobin, platelet count, albumin, lactate dehydrogenase, serum creatinine, serum calcium, β2-microglobulin, etc.), cytogenetic FISH test results t(11;14), del13q, del17p, CCND1, MYD88 L265P, and CXCR4 treatment regimens, and survival outcomes. In this study, 22 patients received initia induction chemotherapy containing proteasome inhibitors (including 5 cases on the PAD regimen, 15 on VCD, and 2 on VD). Ten patients were treated with PIs combined with immunomodulatory drugs (Imids), and 4 patients were in the CD38 monoclonal antibody group (including 2 on Dara+VD, 1 on Dara+RD, and 1 on Dara+VRD). Two patients received other regimens (1 on R-CHOP and 1 on TCD). Among them, 5 patients underwent sequential autologous hematopoietic stem cell transplantation.
Follow-up was conducted until December 2024 via telephone interviews, supplemented by medical records and outpatient data.
Efficacy Evaluation
The treatment response was evaluated using the 2014 IMWG response criteria, which include the following categories: stringent complete response (sCR), complete response (CR), very good partial response (VGPR), partial response (PR), and progressive disease (PD). The overall response rate (ORR) was defined as the sum of responses achieving =PR. Progression-free survival (PFS) was calculated from the start of first-line induction therapy until disease progression, while overall survival (OS) was defined as the time from diagnosis to death or the last follow-up.
Statistical Analysis
Statistical analysis was performed using SPSS 26.0 software. Normally distributed continuous variables were expressed as mean ± standard deviation (x ± s), while non-normally distributed data were presented as median (Q1, Q3). Categorical variables were described as numbers (percentages).
Survival curves were generated using the Kaplan-Meier method, and differences between groups were compared with the log-rank test. Prognostic factors were analyzed using Cox proportional hazards regression models. Variables with P<0.05 in univariate analysis were included in multivariate analysis. A two-sided test was used with a significance level of a=0.05.
Results
Baseline Clinical Characteristics
Age and Gender: IgM-MM cohort (n=44) had a mean age of 63.68 years (range: 36 ~ 82), with male predominance (65.9% vs 34.1% female). Age and Gender: The average age of IgM-MM patients was 63.68 years (range: 36~82), with males accounting for 65.9% and females for 34.1%. Compared to non-IgM-type MM patients, IgMMM patients were older, and the difference was statistically significant (p = 0.003). In contrast, IgM-MM patients were younger than WM patients, and this difference was also statistically significant (p = 0.043) (Table 1 and Table 2).
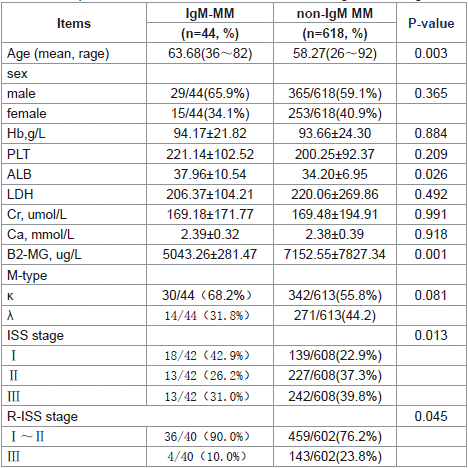
Table 1: Comparison the baseline Clinical Characteristics of IgM and non-IgM MM.
Laboratory Findings: The albumin levels in IgM-MM patients were higher than those in non-IgM MM patients (p = 0.026), while β2-microglobulin levels were lower (p = 0.001). Consequently, a higher proportion of IgM-MM patients were classified as ISS stage I or R-ISS stage I~II.
When comparing IgM-MM with WM patients, IgM-MM patients exhibited higher serum creatinine levels (p= 0.008) and higher serum calcium levels (p= 0.024) (Table 1 and Table 2).
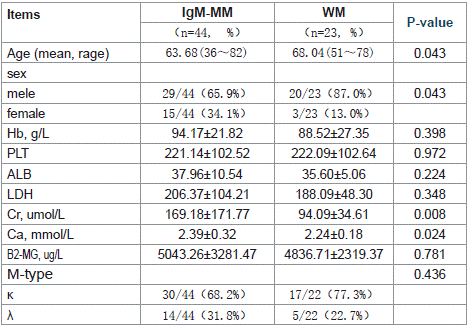
Table 2: Comparison the baseline Clinical Characteristics of IgM and WM.
Cytogenetic Characteristics: The t(11;14) translocation was significantly more prevalent in IgM-MM patients (51.4%, 18/35) compared to non-IgM MM cases (12.6%, 76/601; p<0.001). Flow cytometric analysis revealed markedly higher CD19+ expression in WM patients (83.3%, 15/18) versus IgM-MM (10.5%, 2/19; p=0.006). CCND1 positivity was observed in 29.6% (8/27) of IgM-MM patients but absent in WM (0/16; p=0.040).
Morphologically, bone marrow plasma cells in IgM-MM patients predominantly exhibited plasmacytic differentiation (72.4%, 21/29), whereas WM cases uniformly demonstrated lymphoplasmacytic morphology (100%, 21/21; p=0.009). Molecular analysis showed no MYD88 mutations in IgM-MM patients, contrasting with 65.2% (15/23) mutation positivity in WM (p=0.002) (Tables 3 and 4).
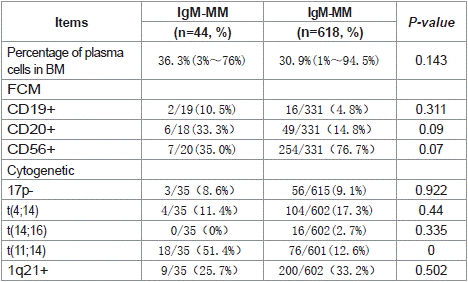
Table 3: Comparison the flow cytometric and cytogenetic of IgM and non-IgM MM.
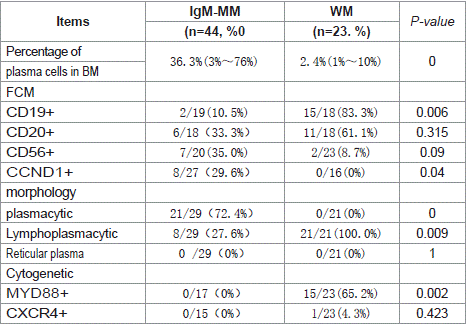
Table 4: Comparison the flow cytometric and cytogenetic of IgM and WM.
Treatment Regimens
Among the 44 IgM-MM patients, 39 received chemotherapy (=1 course), while 5 were untreated. The induction regimens included: Proteasome inhibitor (PI)-based therapy: 50.0% (22/44), PIs combined with immunomodulatory drugs (IMiDs): 25.0% (10/44), CD38 monoclonal antibody-based therapy: 9.1% (4/44), Other regimens: 15.9% (7/44, including 1 R-CHOP, 1 IMiD-containing regimen, and 5 who discontinued treatment), Additionally, 11.4% (5/44) of patients underwent sequential autologous stem cell transplantation (ASCT).
The induction treatment protocols for both IgM and non-IgM MM patients are detailed in Table 5.

Table 5: The induction treatment protocols for both IgM and non-IgM MM patients.
Efficacy Analysis
A comparative analysis of treatment response between IgM and non-IgM multiple myeloma patients following induction chemotherapy revealed: The complete response (CR) rate was 20% (7/35) in IgM-MM versus 33.2% (148/446) in non-IgM MM, with no statistically significant difference (p=0.108).The =very good partial response (=VGPR) rate was 57.1% (20/35) in IgM-MM compared to 71.7% (320/446) in non-IgM MM, again showing no statistically significant difference (p=0.067) (Table 6 and Figure 1).
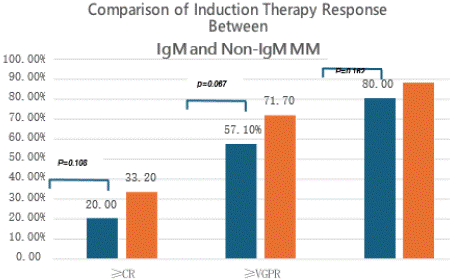
Figure 1: Comparison of Induction Therapy Response Between IgM and
Non-IgM MM.
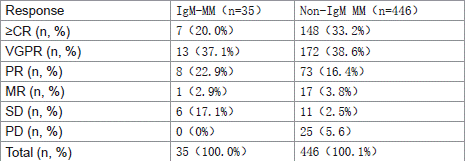
Table 6: Comparison of Induction Therapy Response Between IgM and Non-
IgM MM.
Survival Analysis
Comparison of PFS and OS between IgM and non-IgM MM: Median progression-free survival (PFS) was 42.18 months for IgMMM versus 47.0 months for non-IgM MM (p=0.165). Median overall survival (OS) was 62.79 months for IgM-MM compared to 63.54 months for non-IgM MM (p=0.736). No statistically significant differences were observed in either median PFS or OS between the two groups (Figure 2).
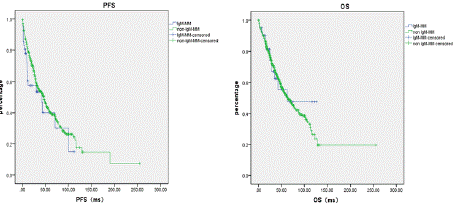
Figure 2: Comparison of PFS and OS between IgM and non-IgM MM.
Impact of Treatment Regimens on Survival Outcomes in IgM-MM: The median progression-free survival (PFS) for different treatment groups was as follows: PI- based regimens: 71.0 months, PI+IMiD combinations: 30.0 months and CD38 monoclonal antibody group: not reached. The difference among groups was not statistically significant (p=0.577). Regarding overall survival (OS), the median OS had not been reached in any treatment group (p=0.186) (Figure 3).
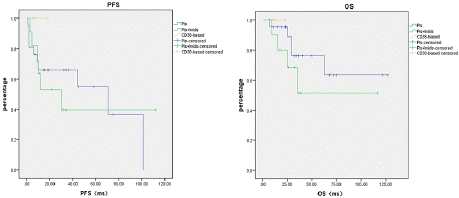
Figure 3: Impact of Treatment Regimens on Survival Outcomes in IgM-MM.
Impact of ASCT on PFS and OS in IgM-MM Patients: Among 44 IgM-MM patients, 5 underwent autologous stem cell transplantation (ASCT). The median PFS was 42.42 months for ASCT recipients versus not reached for non-ASCT patients (p=0.353), while the median OS was 62.80 months versus not reached (p=0.141).
These results suggest that ASCT may improve PFS and OS in IgMMM patients; however, due to the limited sample size, the differences currently lack statistical significance (Figure 4).
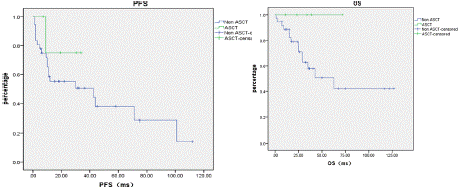
Figure 4: Impact of ASCT on PFS and OS in IgM-MM Patients.
Survival Analysis of IgM-MM with Different Cytogenetic Profiles: Patients with both t(11;14) translocation and CCND1 positivity demonstrated the most favorable prognosis, showing a median PFS of 101.0 months. In comparison, the CCND1- positive group without t(11;14) translocation had a median PFS of 71.0 months. However, due to limited sample size, this difference did not reach statistical significance (p=0.378).
The median OS had not been reached in either group (p=0.216). Survival curve trends indicated that patients with concurrent t(11;14) translocation and CCND1 positivity had the best clinical outcomes (Figure 5).
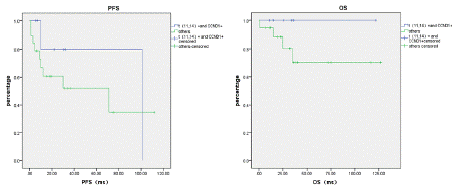
Figure 5: PFS and OS in the t(11,14) translocation and CCND1-positive
group and the non-t (11,14) translocation and CCND1-positive group.
Discussion
Multiple myeloma (MM) is a hematologic malignancy characterized by clonal plasma cell proliferation and excessive monoclonal immunoglobulin production. The IgM-type is exceptionally rare, accounting for only 0.5%~1.0% of all MM cases [1], and demonstrates distinct clinicopathological and genetic features compared to other immunoglobulin subtypes. While Chinese IgM MM cases have previously been limited to case reports, this multicenter study presents the first comprehensive analysis of 44 IgM MM patients in China, providing valuable insights into the disease's clinical characteristics, cytogenetic profile, treatment response, and prognostic factors.
Our results demonstrate that IgM-MM patients typically present at an older age, with higher albumin levels and lower β2-MG levels compared to non-IgM MM cases. Notably, significant genetic differences were observed: the t(11;14) translocation frequency was markedly higher in IgM-MM, suggesting CCND1 overexpression may drive disease progression in this subtype. Due to their shared IgM secretion, IgM-MM and Waldenström macroglobulinemia (WM) often pose diagnostic challenges. The presence of MYD88 mutation serves as a crucial differentiating marker, as it is characteristically found in WM but not in IgM-MM [4].
Our findings demonstrate that IgM MM patients present at an older mean age (63.68 years) compared to non-IgM MM (58.27 years; p=0.003), though younger than Waldenström macroglobulinemia (WM) patients (68.04 years; p=0.043). The IgM MM cohort exhibited significantly higher serum albumin (p=0.026) and lower β2- microglobulin levels (p=0.001) than non-IgM MM, correlating with a greater proportion of early-stage disease by both ISS (42.9% stage I) and R-ISS (90.0% stage I~II) criteria. Notably, IgM MM patients showed elevated serum creatinine (p=0.008) and calcium levels (p=0.024) compared to WM cases.
Waldenström Macroglobulinemia (WM) and IgM Multiple Myeloma (IgM-MM): Key Diagnostic Differences
WM is a lymphoplasmacytic neoplasm characterized by the secretion of IgM monoclonal immunoglobulin. Although its clinical manifestations often overlap with those of IgM-MM, making differential diagnosis challenging, our study identified several distinguishing features:
(1) Bone Marrow Plasma Cell Morphology: IgM-MM: Predominantly plasmacytic morphology WM: Consistently lymphoplasmacytic morphology.
(2) Flow Cytometry (CD19 Expression): WM patients showed significantly higher CD19+ cell proportions than IgM-MM patients (p =0.006).
(3) Genetic Abnormalities: IgM-MM had higher rates of t(11;14) translocation and CCND1 positivity compared to WM (p= 0.040).
(4) MYD88 Mutation Status: MYD88 mutations were consistently absent in IgM-MM but frequently present in WM (p= 0.002).
These findings align with international literature. Schuster et al. [4] reported that WM predominantly carries the MYD88 L265P mutation, whereas MM typically lacks this alteration. Additionally, IgM-MM exhibits a high incidence of IgH translocations, with t(11;14) being the most common (detectable in ~40% of patients) [3,8].
Key Findings in IgM-MM: Genetic Characteristics: The t(11;14) translocation was significantly more frequent in IgM-MM patients (51.4%) compared to non-IgM MM patients (12.6%), with a statistically significant difference (p <0.001). This finding is consistent with previous international reports [8].
Treatment Response: No statistically significant differences were observed between IgM-MM and non-IgM MM patients in terms of complete response (CR) rates or = very good partial response (=VGPR) rates following induction chemotherapy (p = 0.067).
Prognosis and Survival Outcomes: Comparative Survival Analysis (IgM-MM vs Non-IgM MM): Median PFS: 42.18 months (IgM-MM) vs 47.0 months (non-IgM MM) (p=0.165). Median OS: 62.79 months (IgM-MM) vs 63.54 months (non-IgM MM) (p=0.736). No statistically significant differences were observed in either PFS or OS between the two groups.
Genetic Impact on Prognosis in IgM-MM: Patients with concurrent t(11;14) translocation and CCND1 positivity demonstrated superior outcomes: Median PFS: 101.0 months (t(11;14)+/CCND1+) vs 71.0 months (t(11;14)-/CCND1+).The difference did not reach statistical significance (p=0.378), likely due to limited sample size.
Impact of Treatment Modalities on Prognosis: Comparison of Therapeutic Regimens: No statistically significant differences were observed in median PFS or OS among: PI-based regimens (p=0.577 for PFS). PI+IMiD combinations (p=0.186 for OS). CD38 monoclonal antibody therapy. ASCT Outcomes in IgM-MM: Among 44 IgM-MM patients, 5 underwent ASCT: Median PFS: 42.42 months (ASCT) vs not reached (non-ASCT) (p=0.353) Median OS: 62.80 months (ASCT) vs not reached (non-ASCT) (p=0.141) While ASCT demonstrated potential clinical benefits in both PFS and OS, the differences did not reach statistical significance due to limited sample size (n=5). These findings align with international reports [2,5].
Conclusions
IgM multiple myeloma (IgM-MM) is an extremely rare subtype. This study represents the first multicenter analysis of a relatively large Chinese IgM-MM cohort (n=44). Our findings demonstrate that IgM-MM patients typically present at an older age, with earlier ISS/R-ISS stages and a high frequency of t(11;14) translocation, while maintaining overall prognosis comparable to non-IgM MM. The study establishes comprehensive diagnostic criteria to differentiate IgM-MM from Waldenström macroglobulinemia (WM) through: Bone marrow cytomorphology, Flow cytometry, Cytogenetic profiling, MYD88 mutation status. These findings provide valuable insights into the clinical characteristics, cytogenetic features, and treatment responses of IgM-MM in the Chinese population. However, further multicenter prospective studies with larger sample sizes are warranted to better elucidate the pathogenesis of IgM- MM and guide personalized treatment strategies. IgM multiple myeloma (MM) is an exceptionally rare disease subtype. This study represents the first multicenter analysis of a relatively large Chinese IgM MM cohort (n=44), providing important insights into its distinct clinicopathological features.
References
- Kyle RA, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78: 21–33.
- Castillo JJ, et al. IgM myeloma: A multicenter retrospective study of 134 patients J. Am J Hematol. 2017: 746-751.
- Atrash Shebli, et al. Clinical Presentation and Gene Expression Profiling of Immunoglobulin M Multiple Myeloma Compared With Other Myeloma Subtypes and Waldenström Macroglobulinemia [J]. J Glob Oncol. 2018; 4: 1-8.
- Schuster SR, et al. IgM multiple myeloma: disease definition, prognosis, and differentiation from Waldenstrom’s macroglobulinemia. Am J Hematol. 2010.
- Morris Curly, et al. Efficacy and outcome of autologous transplantation in rare myelomas. Haematologica, 2010; 95: 2126-2133.
- BAZARBACHI. IgM-MM is predominantly a pre–germinal center disorder and has a distinct genomic and transcriptomic signature from WM. BLOOD. 2021.
- Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma[J]. Lancet Oncol. 2016; 17: e328-e346.
- Avet-Loiseau H, Garand R, Lodé L, et al. Translocation (t 11;14) (q13;q32) is the hallmark of IgM, IgE, and nonsecretory multiple myeloma variants. Blood. 2003; 101: 1570-1571.