
Special Article - Multiple Myeloma
Ann Hematol Oncol. 2015;2(1): 1018.
Reversible Posterior Leukoencephalopathy Syndrome due to Carfilzomib
Pauff JM1, Ayres KL2, Sochacki AL3, Morgan D1, Strother MK2, Goemann M1 and Warner JL1,4*
1Department of Medicine, Vanderbilt University, USA
2Department of Radiology and Radiological Sciences, Vanderbilt University, USA
3Department of Medicine, Vanderbilt University, USA
4Department of Biomedical Informatics, Vanderbilt University, USA
*Corresponding author: Warner JL, Department of Medicine and Biomedical Informatics, Division of Hematology & Oncology, Vanderbilt University, Nashville, TN 37232, 2220 Pierce Avenue, 777 Preston Research Building, Nashville, TN 37232-6307, USA,
Received: November 21, 2014; Accepted: January 05, 2015; Published: January 07, 2015
Abstract
Carfilzomib is a proteasome inhibitor used in the treatment of relapsed and refractory plasma cell myeloma. We present here a case of Reversible Posterior Leukoencephalopathy Syndrome (RPLS) attributable to Carfilzomib, an adverse side effect that was not reported in studies prior to the approval of this drug for routine clinical use. The patient was a 67 year- old male with relapsed myeloma who received ten total doses of carfilzomib as part of CCyd, 8 of which were at a dose of 36 mg/m2. He subsequently developed a severe headache with neurological deficits that progressed to a profoundly depressed mental status and seizures. His radiologic findings, clinical course and subsequent recovery supported a diagnosis of RPLS. This follows the first reported case in early 2014 associated with carfilzomib, and follows several reported instances with the use of the earlier approved proteasome inhibitor bortezomib. Our observations may serve as a reference point for future examination of the dose-related toxicities of carfilzomib, both alone and in combination with other therapeutic agents in various therapy regimens. We believe this is an important consideration that should be brought to the attention of all physicians and researchers that use this drug.
Keywords: Myeloma; Proteasome inhibitor; Reversible posterior leukoencephalopathy syndrome; Carfilzomib; Bortezomib
Introduction
The use of novel antineoplastic agents has been a mainstay in the treatment of plasma cell myeloma and has had significant positive impact on the course of this disease [1]. In particular, the use of Proteasome Inhibitors (PIs) such as bortezomib (initially approved by the US Food & Drug Administration [FDA] in 2003 for treatment of relapsed plasma cell myeloma, with further approval in 2008 and 2014 for additional indications) and carfilzomib (approved in 2012 for the treatment of relapsed and refractory myeloma previously treated with bortezomib and an immunomodulatory therapy) has further advanced our ability to treat this incurable disease. Despite their therapeutic potential, these agents are not without significant side effects. We have found in a retrospective analysis across multiple institutions that treatment- related complications have become more common over the same period that novel therapies have become more prevalent [2]. Common adverse effects noted in prospective studies of patients treated with bortezomib included gastrointestinal toxicities, reactivation of herpes zoster virus, bone marrow suppression, and dose-limiting peripheral neuropathy that is frequently irreversible [3- 5]. Second generation PIs such as carfilzomib have been shown to be better tolerated, with fewer adverse effects even in those with heavily pretreated disease [6]. There were no apparent adverse effects noted in the central nervous system with the use of either of these PIs in studies conducted prior to their FDA approval.
Reversible posterior leukoencephalopathy syndrome (RPLS, also referred to in the literature as posterior reversible encephalopathy syndrome or PRES) is best described as a clinical syndrome with supportive radiologic findings. Clinical manifestations can range from headache to seizure activity, and corresponding MRI of the brain most commonly reveals a relatively symmetric distribution of edema in the parietal and occipital lobes. It was first recognized by Hinchey and colleagues as a potentially fatal syndrome related to severe hypertension and immunosuppression [7]. It has been welldocumented as a potential adverse event due to many medications, and was recently reported for the first time in the setting of treatment with carfilzomib [8]. We recently took care of a gentleman who developed RPLS within three days of his tenth overall dose of carfilzomib.
Case Presentation
The patient was a 67 year-old white male with hyposecretory IgG plasma cell myeloma with high risk features (TP53 gene deletion by fluorescence in situ hybridization). At the time of diagnosis he had 30-40% plasma cells in the bone marrow, a syndrome of renal failure (eGFR of less than 10), anemia, and lytic bone lesions. His other comorbidities at the time of diagnosis were minimal, except for a diagnosis of hypertension and a remote history of colon cancer (early stage; resected to cure without any adjuvant chemotherapy). He was treated with a single cycle of lenalidomide, bortezomib, and dexamethasone (RVD) in combination at an outside institution. He then transferred care to our institution, and was then treated with a combination of bortezomib and dexamethasone (VD) for three additional cycles, with several doses of bortezomib being omitted due to symptoms of peripheral neuropathy. His renal function normalized. Repeat bone marrow examination indicated complete remission as defined by the International Myeloma Working Group; however, due to his performance status at that time autologous Hematopoietic Cell Transplantation (HCT) was deferred, and he began maintenance lenalidomide. He completed four cycles and was again considered for HCT, but unfortunately had relapse of his disease as noted by 25% plasma cells in the marrow. He then began treatment with a combination of Carfilzomib (20 mg/m2 on days 1 & 2; 36 mg/ m2 on days 8, 9, 15, 16), cyclophosphamide (300 mg/m2 on days 1, 8, 15), and dexamethasone 40 mg weekly (CCyd) on a 28-day cycle [9]. Throughout his treatment courses, he received standard prophylaxis and initially bisphosphonates, which were changed to denosumab due to intolerable side effects, six months prior to beginning CCyd.
His first cycle of CCyd was complicated by otitis media successfully treated with antibiotics. He was without symptoms at the beginning of his second cycle (seventh overall infusion of carfilzomib, the fifth at 36 mg/m2), and the cyclophosphamide dose was reduced to 250 mg/ m2 given his prior otitis. Three days after his eighth overall infusion of Carfilzomib, he developed a severe headache that progressed over several hours to include nausea, emesis, and altered mentation. He eventually became unresponsive to verbal stimuli and was taken to a local emergency department. There, he suffered a tonic-clonic seizure treated successfully with lorazepam. He had no history of prior seizures. His vital signs included a blood pressure of 182/80, heart rate of 49 (which was his baseline bradycardia) and oxygen saturation of 91% by pulse oximetry. Complete blood count, comprehensive metabolic panel with electrolytes, and ammonia were within normal limits. There was no evidence of stroke or intracranial bleeding on a head CT, which revealed only known calvarial changes attributed to his myeloma. He was given hydralazine and fosphenytoin, and later started on levetiracetam. On transfer to our institution, his vital signs were significant for a blood pressure of 159/62. He was somnolent but opened his eyes in response to his name. He could only repeat his first name when asked location and date. He could not participate with a full neurological exam but did withdraw to pain in all four extremities. He also was found to have hyporeflexia throughout his extremities.
MRI of the brain showed hyperintense signal on T2-weighted FLAIR imaging in the cortex and sub cortical white matter of bilateral occipital lobes, which was new when compared to a prior study of approximately 6 months (Figure). Tissue did not have restricted diffusion on Diffusion Weighted Imaging (DWI). Given the pattern of edema, which was posterior and involved both cortex and sub cortical white matter in a non-vascular territory, findings was most consistent with RPLS. RPLS most commonly affects the parietal and occipital lobes with a somewhat symmetric pattern of distribution, as was present in this case, but frontal lobe, inferior temporal-occipital junction, and cerebellum can be involved, in that order. Edema may also be seen in the basal ganglia, brain stem, and deep white matter. The majority of cases do not demonstrate restricted diffusion, indicating that the edema is vasogenic rather than cytotoxic. In a significant minority of cases, between 11-26%, concomitant foci of restricted diffusion may be present representing focal areas of tissue injury [10].
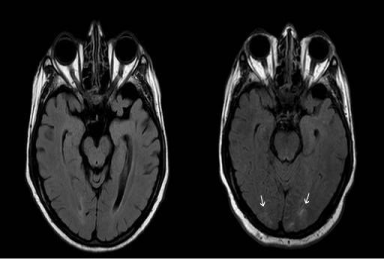
Figure 1: Image on the right represents the current MRI study with patchy
areas of abnormal T2 FLAIR signal in the occipital lobes, new since the prior
exam shown on the left.
His clinical and radiological findings were thus felt to be most consistent with RPLS induced by repeat exposure to carfilzomib. CCyd was discontinued and he was observed briefly in the hospital. There, he had some visual hallucinations including the perception of "tape" covering everything within a visual hemi field. These slowly resolved over 24-48 hours and he was discharged home with routine follow-up. When seen in the outpatient clinic 10 days after his initial presentation, his neurologic recovery was noted to be complete and he was without residual deficits, again supporting his initial diagnosis of RPLS. He was subsequently treated with pomalidomide and dexamethasone, along with continuation of denosumab, and has not had any recurrence of the RPLS symptoms for more than 3 months.
Discussion
This report represents the second instance of this syndrome in the setting of carfilzomib, after a recent report by Cai and colleagues [8]. Although our patient was receiving this drug in combination with dexamethasone and cyclophosphamide, it is unlikely that either of these drugs was the primary culprit. The patient had been taking dexamethasone as an adjunct to multiple regimens without major side effects. Cyclophosphamide has been implicated as a causative agent of RPLS in only a single case report [11], and is a very commonly used drug for a variety of malignant and rheumatologic conditions. However, it is possible that the combination of these three drugs may have had a role in precipitating RPLS and future reports should focus on the degree to which carfilzomib has been used in combination with other agents, or in any regimen using greater than 27 mg/m2/day. There are also several case reports on RPLS attributable to bortezomib, with resolution of neurological symptoms within days of diagnosis and corresponding to discontinuation of the drug [12,13]. Investigation into the mechanisms by which carfilzomib and bortezomib can induce this clinical syndrome is warranted, and clinical observations such as these lend further support to the study of off-target class effects of these agents [14].
The management of RPLS consists of measures aimed at its clinical manifestations. The great variety of clinical settings and reports of RPLS currently make for limited published recommendations in the prevention and treatment of this syndrome. Control of hypertension has been a common approach, given that most patients seem to present with significant elevations above their baseline [7,12,15]. A goal reduction in BP of 10-25% has been suggested (the latter especially in cases of malignant hypertension). Seizures are also a common manifestation of RPLS, and control with phenytoin appears to be a commonly used and effective treatment. Of note, there does not appear to be an elevated long-term risk of seizures in these patients [15].
While various side effects associated with the use of carfilzomib had been reported in studies prior to its FDA approval, this represents an important new toxicity that must be considered and monitored for while treating patients with this second-generation PI.
References
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008; 111: 2516-2520.
- Warner JL, Alterovitz G, Bodio K, Joyce RM. External phenome analysis enables a rational federated query strategy to detect changing rates of treatment-related complications associated with multiple myeloma. J Am Med Inform Assoc. 2013; 20: 696-699.
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005; 352: 2487-2498.
- Orlowski RZ, Nagler A, Sonneveld P, Bladé J, Hajek R, Spencer A, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007; 25: 3892-3901.
- Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004; 127: 165-172.
- Siegel D, Martin T, Nooka A, Harvey RD, Vij R, Niesvizky R, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013; 98: 1753-1761.
- Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 334: 494-500.
- Cai X, Bhattacharyya S, Plitt A, Raibagkar P, Labuzetta JN, Schleicher SM, et al. Management of Posterior Reversible Encephalopathy Syndrome Induced by Carfilzomib in a Patient With Multiple Myeloma. J Clin Oncol. 2014.
- Bringhen S, Petrucci MT, Larocca A, Conticello C, Rossi D, Magarotto V, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014; 124: 63-69.
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008; 29: 1036-1042.
- Abenza-Abildua MJ, Fuentes B, Diaz D, Royo A, Olea T, Aguilar-Amat MJ, et al. Cyclophosphamide-induced reversible posterior leukoencephalopathy syndrome. BMJ Case Rep. 2009.
- Kager LM, Kersten MJ, Kloppenborg RP, Van Oers R, Van den Born BJ. Reversible posterior leucoencephalopathy syndrome associated with bortezomib in a patient with relapsed multiple myeloma. BMJ Case Rep. 2009.
- Ho CH, Lo CP, Tu MC. Bortezomib-induced posterior reversible encephalopathy syndrome: clinical and imaging features. Intern Med. 2014; 53: 1853-1857.
- Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ, et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011; 17: 2734-2743.
- Bakshi R, Bates VE, Mechtler LL, Kinkel PR, Kinkel WR. Occipital lobe seizures as the major clinical manifestation of reversible posterior leukoencephalopathy syndrome: magnetic resonance imaging findings. Epilepsia. 1998; 39: 295-299.