
Special Article - Hematology
Ann Hematol Oncol. 2015;2(1): 1019.
POEMS Syndrome and Small Lymphocytic Lymphoma Co-Existing in the Same Patient: A Case Report and Review of the Literature
Kasi Loknath Kumar A1,2*, Mathur SC3 and Kambhampati S1,2
1Department of Hematology and Oncology, Veterans Affairs Medical Center, USA
2Department of Internal Medicine, University of Kansas Medical Center, USA
3Department of Pathology, Veterans Affairs Medical Center, USA
*Corresponding author: Kasi Loknath Kumar A, Department of Internal Medicine, Division of Hematology and Oncology, University of Kansas Medical Center, Kansas City, 2330 Shawnee Mission Parkway, MS 5003, Suite 210, Westwood, KS, 66205, Kansas, USA
Received: November 25, 2014; Accepted: January 06, 2015; Published: January 08, 2015
Abstract
The coexistence of B-cell Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL) and Plasma Cell Dyscrasias (PCD) has rarely been reported. The patient described herein presented with a clinical course resembling POEMS syndrome. The histopathological evaluation of the bone marrow biopsy established the presence of an osteosclerotic plasmacytoma despite the absence of monoclonal protein in the peripheral blood. Cytochemical analysis of the plasmacytoma demonstrated monotypic expression of lambda (λ) light chains, a typical finding associated with POEMS syndrome. A subsequent lymph node biopsy performed to rule out Castleman's disease led to an incidental finding of B-CLL/SLL predominantly involving the B-zone of the lymph node. The B-CLL population expressed CD19, CD20, CD23, CD5, HLA-DR, and kappa (κ) surface light chains. To the best of our knowledge, a simultaneous manifestation of CLL/SLL and POEMS has not been previously reported in the literature. The expression of a different immunoglobulin (Ig) light chain on the plasmacytoma (λ) and CLL (κ) suggested a biclonal B-cell origin. In our patient, a definite clonal relationship between the two neoplasms by Ig heavy chain gene rearrangements could not be established because of the nonsecretory myeloma and absence of CLL lymphocytosis in the peripheral blood.
Keywords: POEMS; CLL/SLL; Clonality; Gene rearrangement
Abbreviations
VEGF: Vascular Endothelial Growth Factor; TNF-a: Tumor Necrosis Factor-Alpha; IL-1β: Interleukin 1-Beta; IL-6: Interleukin-6; PSA: Prostate Specific Antigen; HIV: human immunodeficiency virus.
Introduction
POEMS is an acronym coined for a rare multisystem plasma cell neoplasm characterized by polyneuropathy, organomegaly, endocrinopathy, Monoclonal paraprotein (M protein), and skin changes [1-4]. Although there are several other associated features that are not included in the acronym, the most consistent clinical feature is that of a chronically progressive peripheral polyneuropathy [1-4]. Another defining element of this entity is the presence of a monoclonal gammopathy, which is usually an IgA-λ or IgG-λ [1- 4]. While the clinical spectrum of Plasma Cell Dyscrasia (PCD) in POEMS is variable, the most common subtype reported in all large series is the Osteosclerotic Myeloma (OSM) [1-4]. The molecular basis for such diverse manifestations seen in POEMS has not yet been defined. However, it is clear that the neoplastic clone of this plasma proliferative disorder expresses a pattern of somatic mutation in the Variable (V)-region genes that have the signature of a neoplasm derived from the post-germinal B-cells [5]. These proliferating monoclonal plasma cells then produce an antibody-mediated attack against neural antigens and secrete inflammatory cytokines, such as VEGF, TNF-a, IL-1β and IL-6 that are directly or indirectly responsible for the pathogenesis of this syndrome [3,4,6-9].
Unlike PCD, the clonal evolution of the most common B-cell derived malignancy Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Leukemia (SLL) has been shown to be remarkably complex. Many groups have published work within the past decade suggesting that CLL is not simply a homogenous disease of slowlyproliferating and self-renewing B cells [10-12]. Rather, CLL, these days, is sub classified on the basis of the mutation status of V genes and the expressions of CD 38 and ZAP-70 [10-12]. Due to differences in the cellular evolution of CLL/SLL and PCD, these cancers rarely coexist in the same individual [10]. In those rare cases where CLL and PCD are coexistent, questions remain as to whether or not these cancers are clonally related. Presently, there are no definite conclusions. In this report we present a patient with POEMS syndrome in which B-CLL/SLL was found to be coexisting. Such a finding of concurrent POEMS and CLL has not been previously reported in literature.
Case Report
A 77-year-old Caucasian male was referred to the Hematology department at the Veteran Affairs (VA) Medical Center in Kansas City, Missouri for the evaluation of lymphadenopathy and osteosclerotic spinal lesions. His past medical history was extensive and most significant for the resection of a pituitary macro adenoma that resulted in panhypopituitarism. In addition, the patient also had a ten year history of progressive Chronic Inflammatory Demyelinating Polyneuropathy (CIDP), which the Electromyography study (EMG) confirmed as an axonal sensor motor polyneuropathy. At the timeof initial evaluation, physical examination showed right posterior cervical and left axillary lymphadenopathy, diminished breath sounds with percussion dullness at the lung bases, and bilateral lower extremity edema. Abdomen was soft and without organomegaly. The patient was wheelchair bound due to an areflexic, grade 4/5 lower extremity weakness. Computed Tomography (CT) scan showed bilateral pleural effusions as well as extensive sclerotic lesions in the thoracolumbar spine, ribs, and pelvis. The results of a technetium bone scan were normal but a metastatic skeletal survey showed diffuse osteosclerosis in the skull and vertebral bodies (Figure 1).
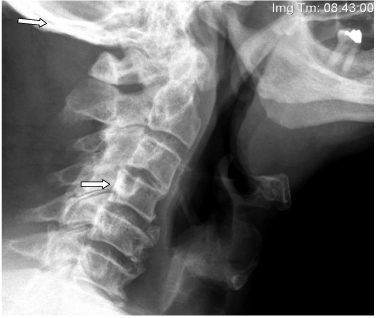
Figure 1: Plain film of the skull and cervical spine showing sclerotic lesions
(see arrows).
Laboratory studies showed a hemoglobin 14.1 g/dL, hematocrit 44.2, MCV 98.2 fl, MCH 31.3 pg, MCHC 31.9 g/L, platelet count 339 x 109/L, leukocyte count 10.3 x 109/L with 54% neutrophils, 36% lymphocytes, 9% monocytes, and 1% eosinophils. The Peripheral Blood (PB) smear showed mild rouleaux formation. Chemistry profile revealed a creatinine of 0.9 mg/dL, calcium of 7.7 mg/dL, albumin of 2.7g/dL, total protein of 5.5 g/dL, and alkaline phosphatase of 56 IU/L. Serum protein electrophoresis and immunofixation failed to show a monoclonal protein. A 24-h urine collection contained 273 mg protein and the urine electrophoresis showed a faint band of monoclonal κ light chain. Serum free κ and λ light chains were elevated at 21.9 mg/L and 68.6 mg/L, respectively, but had a normal ratio of 0.32. Cerebrospinal Fluid (CSF) evaluation was remarkable for an elevated total protein level (944 mg/dL) and CSF-IgG level (771 mg/dL), a pattern often seen in CIDP. The total PSA measured 2.43 ng/mL; interleukin-6 was <10 pg/mL; and HIV serology was negative.
Although plasmacytosis was inconspicuous in the BM aspirate, the histological examination of the BM biopsy showed hypercellularity (50-90%) with osteosclerosis (Figure 2A). Within the area of osteosclerosis was a large collection of plasma cells (plasmacytoma) (Figure 2B). Immunohistochemical (IHC) staining of the core biopsy for κ and λ light chains showed that these plasma cells expressed monoclonal λ (Figure 2C and D). Unexpectedly, the flow cytometry evaluation of the BM aspirate revealed a clonal B-cell CLL population that was positive for CD19, CD20, CD23, CD5, HLA-DR, and also demonstrated weak to moderate monotypic expression of κ surface light chains.
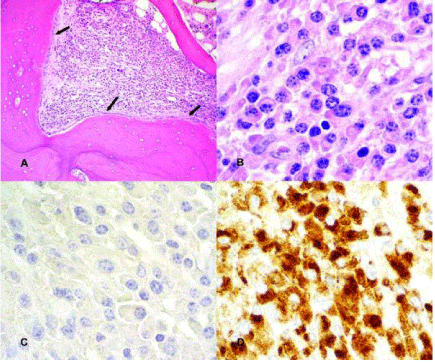
Figure 2: Sclerotic bone with prominent osteoblastic rimming (arrows)
and sheet of plasma cells. (Hematoxylin and eosin, original magnification
x25); B: Plasmacytoma composed of mature plasma cells. (Hematoxylin
and eosin, original magnification x100; C & D: Monoclonal plasma cells,
negative for kappa light chain (C) and positive for lambda light chain (D),
by immunohistochemistry. (Immunoperoxidase, original magnification x100).
Histological examination of a left axillary lymph node excisional biopsy revealed B-cell CLL/SLL, with predominant B-zone involvement (Figure 3A and B). B-CLL/SLL selectively involving the B-zone of the lymph node is an unusual variant [13]. It is morphologically characterized by a deceptively benign pattern at a low magnification due to preserved architecture of the lymph node [13]. Immunohistochemical staining of the lymph node sections showed distinct regions of B-cell (CD20 positive; Figure 3C) and T-cell (CD3 positive; data not presented) zones. The B-cells were uniformly strongly positive for CD5 (Figure 3D) and CD23 (data not shown), supporting the diagnosis of B-CLL/SLL. In order to further elaborate on these findings, a flow cytometric evaluation of the lymph node tissue was done in parallel, which categorically established the diagnosis of a ZAP-70-negative CLL. The B-CLL population expressed CD19, CD20, CD23, CD5, HLA-DR, and κ surface light chains. Analogous to the bone marrow aspirate, the aberrant B-cells in the lymph node expressed weak κ surface light chains. Intravenous Immunoglobulin (IVIG) was administered as treatment for CIDP but there was no neurological recovery noted and the patient remained wheelchair bound. The lymph node biopsy revealed B-CLL/SLL that did not warrant any treatment due to its non-bulky size on presentation. With regards to the POEMS syndrome, cytotoxic treatment was initially withheld due to the patient's poor performance status. During the later course of disease, the patient developed polymicrobial infections, which ultimately resulted in his death.
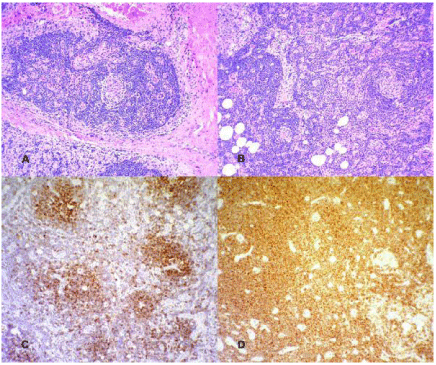
Figure 2: Histological examination of lymph node showing preservation
of vascular architecture and patent sinuses with mild sinus histiocytosis.
Germinal centers are decreased and most show regressive changes with
increased hyalinization and vascularity. The interfollicular regions show
selective and complete replacement of the B-areas with disappearance of
follicles, consistent with a B-zone variant of Chronic Lymphocytic Leukemia
(CLL)/Small Lymphocytic Lymphoma (SLL). (Hematoxylin and eosin, original
magnification x25); C: B-zone pattern of B-cell infiltration, highlighted by
Immunohistochemical staining for CD 20. (Immunoperoxidase, original
magnification x25); D: Immunohistochemical staining for CD5 highlighting
interfollicular T-cells and showing that the B-lymphocytes in the B-zone are
also positive, confirming the diagnosis of B-CLL/SLL. (Immunoperoxidase,
original magnification x 25).
Discussion
In this paper, we report a patient who had a rare presentation of POEMS syndrome coexisting with CLL/SLL. While the acronym POEMS was coined more than two decades ago [1-4], it remained a diagnostic dilemma until recently. Dispenzieri et al proposed unequivocal major and minor criteria to accurately diagnose this obscure syndrome [3,4]. At minimum, two major and one minor criterion are needed to differentiate this syndrome from neuropathy associated with other PCD [3,4]. Despite identifiable monoclonal protein not being present in this patient's serum, a clonal λ plasma proliferative disorder was still conclusively established by histology and cytochemistry of the bone marrow biopsy (Figure 2A-D). As in this case, previous reports have shown that fewer than 10% of POEMS patients will not have any detectable monoclonal protein in the serum or urine, but will reveal biopsy proof of a plasmactyoma that typically has monoclonal λ restriction [3,4]. The diagnosis of POEMS is unquestionable in this patient since he had a non-secretory myeloma with CIDP concomitantly with at least three minor diagnostic criteria comprising of multiple sclerotic bone lesions (Figure 1), pleural effusions, and pedal edema.
T?he pathophysiology of POEMS is poorly understood because it is a rare disease in humans [1-4]. Elevated levels of several cytokines and growth factors have been reported to cause POEMS, most important of them being IL-6 [3,4, 6-9]. Of note, the IL-6 level was low in this patient. Expanding on that finding, we bring attention to the fact that the existing literature has underscored a clinical overlap between the systemic manifestations of POEMS syndrome and Castleman's Disease (CD) in approximately 60% of cases [3,4,6-9]. Mechanistically, there is overwhelming evidence to support the concept that IL-6 overproduction is pathognomonic for such an overlap between POEMS and CD [3,4, 6-9]. Conversely, IL-6 levels have not been found to be increased in POEMS patients without CD [6]. In the case of the patient being reported the histopathological evaluation of the lymph node done to rule out CD, unexpectedly revealed a concomitant B-zone CLL/SLL (Figure 3AD). Furthermore, flow cytometric analysis of the excised lymph node and BM mononuclear cells revealed clonal population of lymphoid B-cells, which was immunophenotypically consistent with CLL/ SLL. Given those findings along with the observation that the IL-6 levels are inconsistently elevated in POEMS syndrome [3,4,6-9], we speculate that the low IL-6 levels measured in our patient could be due to the absence of CD-like changes in the lymph nodes.
The simultaneous manifestation of B-CLL/SLL and PCD is a rarely reported phenomenon [14-26]. In the electronic media, there have been no case reports of coexisting B-CLL/SLL and POEMS in the same patient. Since B-CLL/SLL and PCD are two diverse B-cell malignancies that occur in different stages of B-cell ontogeny, their coexistence understandably raises questions regarding their genetic link. The immunological techniques that have been used to determine the genetic relatedness between B-cell cancers include analysis of the concordance for surface Immunoglobulin (Ig), intra-cytoplasmic Ig expression, and/or the type of M protein secreted in the blood or urine. Results from these studies point to an independent genetically unrelated evolution of B-CLL/SLL and PCD because of the conflicting Ig light chain subtypes [16-21] or dissimilar idiotypes [22,23] expressed by the neoplastic clone of cells. In some, but not all cases, discordant Ig heavy chain isotypes were reported [24-26]. Hence, to further strengthen the argument that heavy chain class switching occurs during B-cell differentiation [24], Fermand et al reported a case in which the B-CLL and plasma cells expressed dissimilar IgG-κ and IgA-κ isotypes, respectively, that shared common idiotypic determinants [25]. Even more intriguing was the finding that CLL lymphocytes, when induced in vitro to differentiate into plasma cells, a heavy chain class switch from IgG to IgA occurred [25]. Despite those reports, there are several shortcomings when heavy and light chain isotypes are used to prove the genetic relatedness of B-cell cancers [10]. To overcome those shortcomings, it is recommended that the clonality of a B-cell population be established by decoding the Ig gene rearrangement pattern of the heavy chain gene, by using either Polymerase Chain Reaction (PCR) or Southern blot technique [10]. As a case example, in a patient with concomitant B-cell CLL/ SLL (IgM-κ) and MM (IgA-λ) described by Saltman et al, the analysis of Ig gene rearrangements was able to detect a common clonal B cell expansion in PB and BM despite differing isotypes and light chain restrictions [26].
As aforementioned, an accurate determination of the genetic relatedness between CLL clone and PCD requires reactivity with an equivalent anti-idiotype antibody, identical Ig Heavy chain (IgH) gene rearrangements, or a similar karyotype [10,16-27]. In our patient, a clonal relationship between the POEMS and coexisting CLL could not be determined by Ig gene configurations in the PB because of the non-secretory type of myeloma and due to absence of PB lymphocytosis. With respect to molecular testing of the BM specimens to detect tumor clones, many of the paraffin samples had been pretreated with B5, a heavy metal fixative that is used to improve cell histology, but inhibits PCR reactions [28]. Nonetheless, by immunophenotyping and cytochemical analysis, the B-lymphocytes stained with an antibody directed against κ chains, whereas the plasmacytoma stained with an anti-λ antibody. As there are several pieces of evidence to suggest that discordance in the expression pattern of heavy and/or light chains cannot be considered as the sole parameter of biclonality [10,24-26], in the patient described, we are cautious in predicting whether both malignancies evolved from a single B-cell progenitor. That being stated based on the incongruent Ig light chain expression, in at least six previous publications, CLL and PCD were considered to be clonally unrelated and their association was a coincidence [16-21]. Taking those discrepancies into consideration, it was our intention to directly examine the clonal relationship of monoclonal Ig's of different isotypes in B-cell clones from freshly-embedded, non-B5 fixed BM samples, using PCR-based assays. Unfortunately, our efforts were unsuccessful as the patient died due to infectious complications before further molecular tissue typing could be performed.
In conclusion, to our knowledge, this is the first report on the occurrence of POEMS and B-CLL/SLL in the same patient. Studies to determine the Ig light chain isotypes demonstrated that in contrast to the λ chain expressing bone marrow plasmacytoma, the CLL lymphocytes stained positive for κ chains. After analyzing the current body of literature, this particular finding leads us to postulate that in this patient the POEMS and CLL originated from different B-cell progenitors.
References
- Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein and skin changes: The P.O.E.M.S. syndrome. Report on two cases and a review of the literature. Medicine (Baltimore). 1980; 59: 311-318.
- Miralles GD, O'Fallon JR, Talley NJ . Plasma-cell dyscrasia with polyneuropathy. The spectrum of POEMS syndrome. N Engl J Med. 1992; 327: 1919-1923.
- Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Therneau TM, Larson DR, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003; 101: 2496-2506.
- Dispenzieri A, Gertz M. POEMS syndrome. Orphanet Encyclopedia. 2005.
- Sahota SS, Garand R, Mahroof R, Smith A, Juge-Morineau N, Stevenson FK, et al. V(H) gene analysis of IgM-secreting myeloma indicates an origin from a memory cell undergoing isotype switch events. Blood. 1999; 94: 1070-1076.
- Gherardi RK, Belec L, Fromont M, Divine M, Malapert D, Gaulard P, et al. Elevated levels of interleukin-1β (IL-1β) and IL-6 in serum and increased production of IL-1β mRNA in lymph nodes of patients with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (POEMS) syndrome. Blood. 1994; 83: 2587-2593.
- Hitoshi S, Suzuki K, Sakuta M. Elevated serum interleukin-6 in POEMS syndrome reflects the activity of the disease. Intern Med. 1994; 33: 583-587.
- Soubrier MJ, Dubost JJ, Sauvezie BJ. POEMS syndrome: a study of 25 cases and a review of the literature. French Study Group on POEMS Syndrome. Am J Med. 1994; 97: 543-553.
- Emile C, Danon F, Fermand JP, Clauvel JP. Castleman disease in POEMS syndrome with elevated interleukin-6. Cancer. 1993; 71: 874.
- Foon KA, Thiruvengadam R, Saven A, Bernstein ZP, Gale RP. Genetic relatedness of lymphoid malignancies. Transformation of chronic lymphocytic leukemia as a model. Ann Intern Med. 1993; 119: 63-73.
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005; 352: 804-815.
- Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004; 351: 893-901.
- Carbone A, Pinto A, Gloghini A, Volpe R, Zagonel V. B-zone small lymphocytic lymphoma: a morphologic, immunophenotypic, and clinical study with comparison to "well-differentiated" lymphocytic disorders. Hum Pathol. 1992; 23: 438-448.
- Pines A, Ben-Bassat I, Selzer G, Ramot B. Transformation of chronic lymphocytic leukemia to plasmacytoma. Cancer. 1984; 54: 1904-1907.
- Makower D, Venkatraj U, Dutcher JP, Wiernik PH. Occurrence of myeloma in a chronic lymphocytic leukemia patients after response to differentiation therapy with interleukin-4. Leuk Lymphoma. 1996; 23: 617-619.
- Kough RH, Makary AZ. Chronic lymphocytic leukemia (CLL) terminating in multiple myeloma: report of two cases. Blood. 1978; 52: 532-536.
- Pedersen-Bjergaard J, Petersen HD, Thomsen M, Wiik A, Wolff-Jensen J. Chronic lymphocytic leukaemia with subsequent development of multiple myeloma. Evidence of two B-lymphocyte clones and of myeloma-induced suppression of secretion of an M-component and of normal immunoglobulins. Scand J Haematol. 1978; 21: 256-264.
- Zalcberg JR, Cornell FN, Ireton HJ, McGrath KM, McLachlan R, Woodruff RK, et al. Chronic lymphatic leukemia developing in a patient with multiple myeloma: immunologic demonstration of a clonally distinct second malignancy. Cancer. 1982; 50: 594-597.
- Jeha MT, Hamblin TJ, Smith JL. Coincident chronic lymphocytic leukemia and osteosclerotic multiple myeloma. Blood. 1981; 57: 617-619.
- Patriarca F, Gaidano G, Capello D, Zaja F, Fanin R, Baccarani M. Occurrence of multiple myeloma after fludarabine treatment of a chronic lymphocytic leukemia: evidence of a biclonal derivation and clinical response to autologous stem cell transplantation. Haematologica. 2000; 85: 982-985.
- Aktan M, Akkaya A, Dogan O, Dincol G . Chronic lymphocytic leukemia and multiple myeloma in the same patient: case report. Leuk Lymphoma. 2003; 44: 1421-1424.
- Brouet JC, Fermand JP, Laurent G, Grange MJ, Chevalier A, Jacquillat C, et al. The association of chronic lymphocytic leukaemia and multiple myeloma: a study of eleven patients. Br J Haematol. 1985; 59: 55-66.
- Hoffman KD, Rudders RA. Multiple myeloma and chronic lymphocytic leukemia in a single individual. Arch Intern Med. 1977; 137: 232-235.
- Preud'homme JL, Seligmann M. Surface bound immunoglobulins as a cell marker in human lymphoproliferative diseases. Blood. 1972; 40: 777-794.
- Fermand JP, James JM, Herait P, Brouet JC. Associated chronic lymphocytic leukemia and multiple myeloma: origin from a single clone. Blood. 1985; 66: 291-293.
- Saltman DL, Ross JA, Banks RE, Ross FM, Ford AM, Mackie MJ. Molecular evidence for a single clonal origin in biphenotypic concomitant chronic lymphocytic leukemia and multiple myeloma. Blood. 1989; 74: 2062-2065.
- Kaufmann H, Ackermann J, Nösslinger T, Krömer E, Zojer N, Schreiber S, et al. Absence of clonal chromosomal relationship between concomitant B-CLL and multiple myeloma--a report on two cases. Ann Hematol. 2001; 80: 474-478.
- Tbakhi A, Totos G, Pettay JD, Myles J, Tubbs RR. The effect of fixation on detection of B-cell clonality by polymerase chain reaction. Mod Pathol. 1999; 12: 272-278.