
Special Article - Hematology
Ann Hematol Oncol. 2015;2(1): 1020.
Acute Myeloid Leukemia in a Patient with Shwachman- Diamond Syndrome: Complex Cytogenetics Redefined
G Choi1*, S Snijder2 and S S Zeerleder1,3
1Department of Hematology, Academic Medical Center, University of Amsterdam, Netherlands
2Department of Clinical Genetics, Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands
3Department of Immunopathology, Sanquin Research and Landsteiner Laboratory of the AMC, Amsterdam, Netherlands
*Corresponding author: G Choi, Department of Hematology, University Medical Center Groningen Hanzeplein 19713 GZ Groningen, The Netherlands
Received: December 04, 2014; Accepted: January 05, 2015; Published: January 07, 2015
Clinical Image
Shwachman-(Bodian)-Diamond Syndrome (SDS) is a rare autosomal recessive disorder (OMIM #260400): A homozygous or compound heterozygous mutation in the SBDS gene causes karyotypic instability, predisposing hematopoietic cells to malignant transformation. We here present a 21-year-old male patient with SDS who has had chronic leukopenia and thrombocytopenia since 4 years before presenting with pancytopenia. Bone marrow aspirate showed massive infiltration with myeloid blasts (Figure, left panel). Cytogenetic analysis showed highly complex chromosomal abnormalities: 97~101, XXYY,-3,-5,+6,+6,+7,-8,-9,- 11,-18,+22,+7~13mar [cp6] / 46,XY [4] (Figure, right panel) with FISH revealing KMT2A (MLL) amplification (11q23) (Figure inset). The diagnosis of poor risk acute myeloid leukemia was made. The patient was treated with high-dose chemotherapy followed by a Stem Cell Transplantation (SCT) from an unrelated donor. There are ongoing discussions whether SCT should be recommended to young adults with SDS before malignant transformation. It remains to be established whether the benefits of SCT outweigh the risk of treatment-related complications.
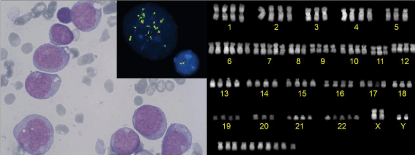
Figure 1: Giemsa stain, showing irregularly shaped myeloid blasts with
monocytic morphology (left panel); karyogram showing multiple chromosomal
abnormalities and hypertetraploidy (right panel); malignant cells had >20
signals for KMT2A vs 2 signals in the normal cell (inset).