
Case Report
Ann Hematol Oncol. 2015;2(5): 1039.
The Rare Occurrence of the Translocation t(8;14) in Chronic Lymphatic Leukemia: Case Report and Review of the Literature
Coym A*, Janjetovic S, Bokemeyer C and Fiedler W
Department of Oncology and Hematology, University Medical Center Hamburg-Eppendorf, Germany
*Corresponding author: Coym A, Department of Oncology and Hematology, BMT with Section of Pneumology, Hubertus Wald Tumorzentrum - University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistrasse 52, 20246 Hamburg, Germany
Received: April 13, 2015; Accepted: May 29, 2015; Published: June 03, 2015
Abstract
Genomic aberrations in Chronic Lymphatic Leukemia (CLL) are important prognostic factors of disease progression and survival and contribute to the treatment decisions and follow-up. The most common chromosomal aberrations detected in CLL are deletions of 13q14, 11q22, 17p13 and trisomy 12. Deletions 17p13.1 and 11q22.3 are strong predictors of poor survival. We report on a rare case of CLL exhibiting the MYC translocation t(8,14)(q24;q32), which is a consistent cytogenetic finding of Burkitt lymphoma. This translocation in CLL mostly appears with other chromosomal abnormalities and is associated with more aggressive disease behavior. After review of the literature, the outcome of CLL patients with MYC translocation is discussed, where prognosis is often poor.
Keywords: Translocation t(8;14); Chronic lymphatic leukaemia; Prognosis
Introduction
CLL is the most common type of leukemia in the western world and it mostly affects elderly persons, with an increasing risk proportional to age [1]. Cytogenetic analysis of CLL cells provides important diagnostic, clinical, and prognostic information, which contribute to the treatment decisions [2-4].
The most common chromosomal aberrations in CLL are trisomy 12, and deletions of: 13q, 11q, 6q, 14q and 17p [2,5,6]. It is known that 17p13.1 or 11q22.3 deletions are associated with a poor prognosis [3,5]. Translocations t(8;14)(q24.1;q32), t(8;22)(q24.1;q11), and t(2;8)(p12;q24.1), known as typical and consistent chromosomal abnormalities of Burkitt lymphoma [7], are rare events in CLL patients and are thought to be associated with poor prognosis [8,9].
Case Presentation
An 83 years old male was admitted to the hospital with a deteriorating general condition followed by presyncope with dizziness and weakness while walking. Furthermore, he complained about weight loss and fever. Routine blood investigation showed leukocytosis (40x109/l with a lymphocytosis of 5.33x109/l), anemia (8,7g/dl), and thrombocytopenia (53x109/l). An increased CRP (32mg/l) and relevant renal insufficiency (creatinine: 1,8mg/dl) were detected as well. Besides splenomegaly, physical examination showed no other abnormalities, especially no enlarged peripheral lymph nodes. Computer Tomography (CT) scan revealed enlarged mesenteric lymph nodes, in addition to splenomegaly.
Cytology of bone marrow aspiration smears showed large blast like cells, besides a small population of mature lymphocytes (Figure 1). Multiparameter flow cytometry revealed expression of CD19, CD20, CD23, CD5, CD79b antigens, implicating the diagnosis of CLL. Even though cytology showed large blast like cells flow cytometry was compatible with CLL e.g. typical B-ALL antigens such as CD10, CD34 and CD13 were not expressed.
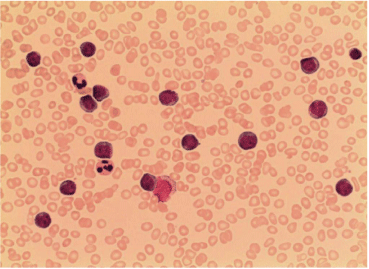
Figure 1: Cytology of bone marrow aspirate smears showed mostly large
blast like cells, besides a small population of mature lymphocytes.
Chromosome banding analysis showed a complex hyperdiploid karyotype with numerous numerical and structural chromosomal aberrations: 48XY, der (1;3)(q10;q10), t(8,14)(q24;q32), +18 (Figure 2).
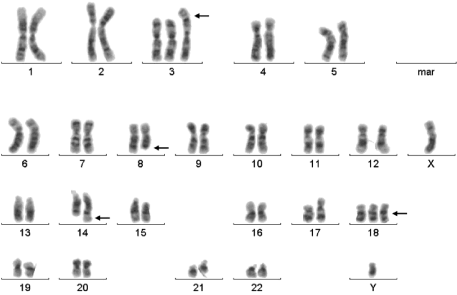
Figure 2: Chromosome banding analysis showing a complex hyperdiploid
karyotype with numerical and structural chromosomal aberrations: 48XY, der
(1;3)(q10;q10), t(8,14)(q24;q32), +18.
Considering the morphological, clinical and immunophenotypic findings, CLL with a rare chromosomal aberration, t(8,14)(q24;q32), was diagnosed. Disease was staged as Binet C. Shortly after diagnosis, the leukocyte count rapidly increased to 55x109/l, with elevated prolymphocyte-levels. Persistent thrombocytopenia was an indication for initiating chemotherapy. Therefore, treatment with Rituximab and Chlorambucil was started. Due to pancytopenia and persisting bone marrow infiltration after two cycles, chemotherapy was changed to Rituximab and Bendamustin. The patient received 4 cycles of the latter combination. After two cycles bone marrow aspiration was performed, showing a complete response; additionally the patient became transfusion independent. Seven months after diagnosis our patient is still in complete remission.
Discussion
Genomic aberrations in CLL are important independent predictors of disease progression and survival and contribute to the treatment decisions [2-4].
The t(8;14)(q24.1;q32) involving MYC and its variants, t(8;22) (q24.1;q11), t(2;8)(p12;q24.1), known as consistent genetic abnormalities of Burkitt lymphoma [7], are rare events in CLL patients. According to the largest series published, these translocations occur in less than 1% of all CLL patients [9]. Moreover, MYC translocations in CLL occur mostly together with other cytogenetic abnormalities with unfavorable prognostic input [9]. They are rarely found as a sole cytogenetic aberration [8]. It has been shown that the prevalence of del (17p) and del (11q), both with negative prognostic impact in CLL, was higher in the MYC translocation cohort than generally observed in CLL [9]. This may suggest a combination effect of MYC translocation with loss of TP53 (del 17p) or ATM (del 11q), in the progression of CLL resulting in a more aggressive disease [8,9]. Patients exhibiting solely MYC translocation may present with less aggressive disease [8].
Furthermore, increased numbers of prolymphocytes seem to be a consistent morphological feature in CLL patients with MYC translocation [9]. The percentage of prolymphocytes may vary widely from 10% to 92% [9].
Cytomorphological variants in patients with CLL are the classic type, mixed type (<10% prolymphocytes) and prolymphocytic leukaemia. Interestingly Jain et al. [10] report on CD5+ high grade lymphomas with four different morphological variants including immunoblasts, centroblasts and giant cells describing a more aggressive clinical course. Jain et al state that CD5 expression alters B-cell behaviour which leads to a more aggressive disease. The pathomechanism remains unclear, but seems to be correlated with complex chromosomal aberrations. So far the influence of the MYC gene has not been studied in this regard.
Reviewing the literature there are 10 published patients [8,11-15] with CLL and the presence of a t (8;14) (Table 1 and 2). Eight out of the ten cases had elevated prolymphocyte levels. In two cases no data are available.
Reference
Gender
Age
WBC
(x109/l)
Hb
(g/dl)
PLC
(%)
Flow cytometry
Cyto-genetics
Therapy
Outcome
Mark et al. [11]
F
88
years
8,5
10,5
31
PB:
CD5, CD20, CD23
PB:
46 XX, der(19)t(1;19)(q23;p13), t(8;14)(q24;q32)[4] /46XX[23]
None
Stable, at least 3 months after diagnosis
Reddy et al. [13]
M
54
years
70
13,1
nd
BM:
CD5 CD20, CD23
BM:
46 XY, t(8;14)(q24.1;q32), -18, +mar[3]/46XY[27]
Rituximab,
Fludarabine,
Dactino-mycine
nd
Amarillo et al. [15]
M
67
years
23
nd
nd
PB:
CD5 CD20, CD23
PB:
46 XY, t(8;14)(q24.1;q32)[6] /46XY[6]
nd
nd
Lakshmaiah et al. [14]
M
65
years
82
8,1
nd
BM:
CD5 CD20, CD23
BM:
45 XY, t(8;14)(q24;q32), -13, add(17)(p11) / 46XY
Chlorambucil
CR, alive at least 72 months after diagnosis
Lau et al. [11]
M
56
years
28
8,9
15
BM:
CD5, CD19, CD23
BM:
47 XY, t(8;14)(q24.1;q11.2), +12,t(14;18)(q32;q21)
Rituximab,
Fludarabine,
Cyclophos-phamide
CR, alive at least
36 months after diagnosis
Huh et al. [8]
M
74
years
81
9,7
20
BM:
CD5, CD19,
CD20, CD23
BM:
46XY, t(8;14)(q24.1;q32)[10]/46XY[10]
Rituximab,
Fludarabine,
Cyclophosphamide
CR, alive at least 26months after diagnosis
second lymphoma:
EBV+
DLBCL
Table 1: Previously published cases of patients with a CLL and t(8;14).
Reference
Gender
Age
WBC
(x109/l)
Hb
(g/dl)
PLC
(%)
Flow cytometry
Cyto-genetics
Therapy
Outcome
Huh et al. [8]
F
72
years
7
12,2
46
BM:
CD5, CD19, CD20, CD23
BM:
46XX, t(8;14)(q24.1;q32)[10]/46XX[22]
R-CHOP (Rituximab, Cyclophosphamide, Vincristine, Doxorubicine, Prednisone)
CR, alive at least 64 months after diagnosis
Huh et al. [8]
M
49
years
73
12,9
15
BM:
CD5, CD19, CD20, CD23
BM:
42-43XY,-2,-3, del(3)(p14),add(4)(q35),-8, t(8;14)(q24.1;q32),-15,-17,-18 add(21)(p11.2),+1-2mar[cp13]/ 46XY[7]
Fludarabine,
Cyclophosphamide,
Hyper-CVAD
(Cyclophosphamide, Vincristine, Cytarabine, Doxorubicine)
Died 9 months after diagnosis
Huh et al. [8]
M
67
years
99
8,9
30
BM:
CD5, CD19, CD23
BM:
48Y,der(X)t(X;1),(p22;q11), add(2)(q37), +7,der(8) t(8;14) (q24;q32), +i(12)(p11), der(14)t(8;14)(q24;q32) add(14)(p12), add(22)(p10)[9]/ 48idem,-14,+mar[3]/47-48idem, -16,+mar[cp4]/ 46XY[1]
Fludarabine,
Cyclophosphamide
Died 105 months after diagnosis
Huh et al. [8]
M
66
years
2
13
10
BM:
CD5, CD19, CD23
BM:
47XY,t(9;10)(q34;q24),+12[4]/ 45X,-Y,-4,t/8;14)(q24.1;32), t(9;10)(q34;q24), add(17) (p13), add(19)(p13.3)[8] 87-90XXY,-Y,-4,-4-t(8;14)(q24.1;q32), t(9;10)(q34;q24)x2, -15,+add(17) (p13)x2,-19,-22, +4-5mar[cp2]/ 46XY[6]
Rituximab,
Fludarabine,
Cyclophosphamide, Campath, Ofatumumab, OFAR (Oxaliplatin, Fludarabine, Alemtuzumab, Rituxan)
Died 49 months after diagnosis
BM: Bone Marrow; CR: Complete Remission; DLBCL: Diffuse Large B-Cell Lymphoma; Hb: Hemoglobine; nd: no data; PB: Peripheral Blood; PLC: Prolymphocytes; WBC: White Blood Cell
Table 2: Previously published cases of patients with a CLL and t(8;14).
One case with transformation into a high grade lymphoma was described [8].
Of the eight published cases with follow-up information three patients had died 9, 49 and 105 months after diagnosis. Five patients were still alive at follow-up between 3 and 72 months.
Here we report the case of a CLL patient exhibiting a complex karyotype including a t(8;14)(q24.1;q32). Our patient developed rapid leucocytosis with increased count of prolymphocytes in peripheral blood, indicating an aggressive clinical course of the disease. So far our patient is doing well 7 months after diagnoses due to therapy with Rituximab and Bendamustin. MYC translocations are rare in CLL, but they are associated with an aggressive clinical course requiring early therapy and should be assumed as a potential negative prognostic predictor.
References
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005; 352: 804-815.
- Dierlamm J, Michaux L, Criel A, Wlodarska I, VandenBerghe H, Hossfeld DK. Genetic abnormalities in chronic lymphocytic leukemia and their clinical and prognostic implications. Cancer Genet Cytogenet. 1997; 94: 27–35.
- Döhner H, Stilgenbauer S, Brenner A. Genomic aberrations and survival in chronic lymphocytic leukemia, N Engl J Med. 2000; 343: 1910-1916.
- Van Den Neste E, Robin V, Francart J, Hagemeijer A, Stul M, Vandenberghe P, et al. Chromosomal translocations independently predict treatment failure, treatment-free survival and overall survival in B-cell chronic lymphocytic leukemia patients treated with cladribine. Leukemia. 2007; 21: 1715-1722.
- Greipp PT, Smoley SA, Viswanatha DS, Frederick LS, Rabe KG, Sharma RG, et al. Patients with chronic lymphocytic leukaemia and clonal deletion of both 17p13.1 and 11q22.3 have a very poor prognosis. Br J Haematol. 2013; 163: 326-333.
- Stevens-Kroef M, van den Berg E, Olde Weghuis D, Geurts van Kessel A, Pfundt R, Linssen-Wiersma M, et al. Identification of prognostic relevant chromosomal abnormalities in chronic lymphocytic leukemia using microarray-based genomic profiling. Molecular Cytogenetics. 2014; 7: 3.
- Diebold J, Jaffe ES, Rafael M, Warnke RA. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of Hematopoietic and Lymphoid Tissues. IARC Press, Lyon, France. 2001.
- Huh YO, Lin KI, Vega F, Schlette E, Yin CC, Keating MJ, et al. MYC translocation in chronic lymphocytic leukaemia is associated with increased prolymphocytes and a poor prognosis. Br J Haematol. 2008; 142: 36-44.
- Put N, Van Roosbroeck K, Konings P, Meeus P, Brusselmans C, Rack K, et al. Chronic lymphocytic leukemia and prolymphocytic leukemia with MYC translocations: a subgroup with an aggressive disease course. Ann Hematol. 2012; 91: 863-873.
- Jain P, Fayad LE, Rosenwald A, Young KH, O’Brian S. Recent advances in de novo CD5+ diffuse large B cell lymphoma. Am J Hematol. 2013; 88: 798-802.
- Lau LC, Lim P, Lim YC, Teng LM, Lim TH, Lim LC, et al. Occurrence of trisomy 12, t(14;18)(q32;q21), and t(8;14)(q24.1;q11.2) in a patient with B-cell chronic lymphocytic leukemia. Cancer Genet Cytogenet. 2008; 185: 95-101.
- Mark HFL, Sotomayor E, Mega A. Occurence of both t(1;19) and t(8;14) in a Patient with Chronic Lymphocytic Leukemia. Exp Mol Pathol. 1999; 66: 238-242.
- Reddy K, Satyadev R, Bouman D, Hibbard MK, Lu G, Paolo R. Burkitt t(8;14) (q24;q32) and cryptic deletion in a CLL patient: report of a ase and review if literature. Cancer Genet Cytogenet. 2006; 166: 12-21.
- Lakshmaiah K, Tejinder S, Kumari P, Gowri MM, Santeesh CT, Lokanatha D, et al. Significance of t(8:14) in CLL?. Indian J Hum Genet. 2010; 16: 102-103.
- Amarillo I, Bui PH, Kantarci S, Rao N, Shackley BS, Garcia R, et al. Atypical rearrangement involving 3’-IGH@ and a breaktpoint at least 400kb upstream of an intact MYC in a CLL patient with an apparently balanced t(8;14)(q24.1;q32) and negative MYC expression. Molecular Cytogenetics. 2013; 6: 5.