
Review Article
Ann Hematol Oncol. 2015;2(6): 1046.
Risk Stratification in Multiple Myeloma
Rajshekhar C and Shaji K*
Division of Hematology, Mayo Clinic, USA
*Corresponding author: Shaji Kumar, Division of Hematology, Mayo Clinic, 200 First Street SW, Rochester, MN 55906, USA
Received: March 30, 2015; Accepted: June 05, 2015; Published: June 10, 2015
Abstract
Multiple myeloma (MM) is a malignant plasma cell disorder, characterized by bone marrow infiltration with clonal plasma cells (PCs), and affects nearly 20,000 patients in United States (US) each year. Revised International Myeloma Working Group (IMWG) diagnostic criteria for MM has included biomarkers, namely, clonal bone marrow PCs = 60%, serum free light chain ratio = 100 and = 1 focal lesion on magnetic resonance imaging (MRI) in addition to traditional CRAB (Hypercalcemia, Renal insufficiency, Anemia and Bone lesions) for defining MM. In the era of risk-adapted therapy with novel agents, importance of comprehensive risk stratification models, including a conglomerate of host factors, tumor factors and factors arising due to host-tumor interaction, is paramount. In this review, we have discussed host factors, including patient demographics and performance status, tumor factors including, albumin, C-reactive protein, lactate dehydrogenase, serum free light chain assay, complete blood count, bone marrow morphology, cytogenetics, gene expression profiling, immunophenotyping and proliferative capacity and factors related to tumor-host interaction, including β-2 microglobulin and renal function, which are important components of risk stratification. Furthermore, response to therapy, including impact of complete remission, early relapse and minimal residual disease after therapy have been shown to predict survival in MM. Clinical application of these components have been reflected in novel risk stratification models, including Mayo stratification of myeloma and risk adapted therapy (mSMART), IMWG and Intergroupe Francophone du Myélome (IFM), with further studies on identifying molecular characteristics of PCs in MM currently underway.
Keywords: Proteasome inhibition; Relapsed myeloma; Stratification
Introduction
Multiple myeloma (MM) is a malignant plasma cell disorder, accounting for 10% of all hematologic malignancies [1]. The diagnosis of MM requires either 10% or more clonal plasma cells in bone marrow along with evidence of end-organ damage or 60% or more clonal plasma cells in the absence of end-organ damage [2]. Historically, the first case of MM was documented by Solly in 1844 in a 39 year old female presenting with fatigue and multiple fractures [3]. Cardinal clinical manifestations of MM attributable to the plasma cell clone are usually described using the acronym CRAB- Calcium elevation, Renal insufficiency, Anemia and Bone disease [4]. Estimated number of new cases of MM in 2014 in the United States (US) was 24,050 and estimated number of deaths being 11,090 [5]. The average ageadjusted incidence of MM in the US is approximately 4 per 100,000, with median age at diagnosis being 65 years [2]. The annual incidence in Europe is 4.5-6 per 100,000 with median age at diagnosis being 65-70 years [6]. In a study conducted in US on the incidence of MM in Olmsted County, Minnesota, the overall annual incidence rate was found to be pretty stable in the last 6 decades [7]. A population based study in Sweden has shown a temporal improvement in median Overall Survival (OS) from 24.3 to 56.3 months in younger (= 65 years) patients with MM diagnosed from 1950 to 2005 [8]. Another larger study using Surveillance, Epidemiology, and End Results (SEER) database showed improvement in five-year Relative Survival rate (RS) during the time period 2004-2011 compared to 1991-2002 in all age-groups, implicating improved survival after approval of novel proteasome inhibitor bortezomib in 2003 [9]. Among patients with relapsed MM after Stem Cell Transplantation (SCT), those relapsing after year 2000 were found to have better OS compared to those before 2000 [10]. All these studies point towards improving survival in MM with advent of novel therapeutic agents in recent decades. Since riskadapted therapy is the standard of care in MM currently [2], the need for a comprehensive risk stratification model for prognostication and assisting with therapeutic decision-making is paramount.
MM is characterized by clinical and biological heterogeneity, with recent genetic analyses identifying subgroups with predictable prognosis across different types of treatment [11]. Various immunoglobulin gene translocations and chromosomal anomalies have been identified in addition to traditional prognostic factors like β-2 microglobulin, which has necessitated chromosomal studies to be conducted for front-line risk stratification and therapeutic consideration in MM [12,13]. Furthermore, data show that novel therapeutic agents like proteasome inhibitors and immunomodulators are more effective than traditional chemotherapy in patients with high-risk cytogenetics [14], advocating the use of such agents for induction prior to SCT in high-risk transplant-eligible patients [2,6]. Due to such heterogeneity in pathogenesis and response to therapy, selection of a risk-stratification tool depends upon the context of host and tumor factors, host-tumor interactions and therapeutic considerations [15]. The future of myeloma therapy lies in precision medicine due to improved understanding of MM cell biology and will require utilization of new relevant prognostic factors [16].
This comprehensive review outlines prognostic markers inherent to patients (host factors), the tumor (myeloma-related factors) and host-tumor interaction (Figure 1). The clinical value of these markers in the era of novel therapeutic agents and transplantation has been described along with current clinical application of evidence-based strategies in prognostication and individualizing treatment.
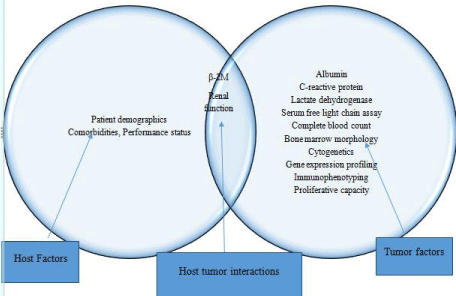
Figure 1: Factors involved in risk stratification.
Host Factors
Patient demographics
Age
Age is an important prognostic marker in MM [15]. Various studies have demonstrated worsening outcomes in MM with advancing age, and the etiology is considered to be multifactorial, including both biological and psychosocial factors [17]. A survival analysis of greater than 10,000 patients from the International Myeloma Working Group (IMWG) showed significantly longer median Overall Survival (OS) in younger patients (<50 years), both after conventional and high dose therapy [18]. Younger patients were found to have lesser frequency of adverse prognostic factors, including high C - reactive protein (CRP), low hemoglobin, increased serum creatinine and poor Performance Status (PS) as well as low International Staging System (ISS) and Durie-Salmon stage. A study on 2316 MM patients younger than 65 years found age >60 years to be significantly associated with shorter OS in those treated with HDT [19]. Interestingly, there was no difference in incidence of high-risk cytogenetics on further subgroup analysis, but β-2 microglobulin levels were higher in patients >65 years of age. Another study conducted by Nordic Myeloma Study Group (NMSG) showed prolonged survival in patients less than 60 years receiving high-dose therapy (HDT) followed by autologous SCT (ASCT) [20]. However, there is conflicting evidence on biological distinction of MM occurring in different age groups. A study on 356 previously untreated MM patients showed similar initial clinical and laboratory characteristics as well as response to therapy in all age groups [21]. Multivariate analysis in a study on MM patients >65 years of age has identified high percentage of S-phase bone marrow plasma cells to be the most powerful independent prognostic factor, indicating the importance of proliferative activity of clonal plasma cells in this age group [22]. A few studies have shown the incidence of chromosomal abnormalities to be similar in MM patients of different age groups, indicating that difference in survival could be due to psychosocial factors and co-morbidities alone [23,24]. A study on MM patients undergoing HDT followed by ASCT did not show age as a significant prognostic marker for both overall and event-free survival [25].
A scoring system based on geriatric assessment has been developed, categorizing elderly (>65 years) patients with MM into 3 groups, “fit”, “unfit” and “frail”. 18 month OS was found decrease from 92% in “fit” group to 73% in “frail” group in Cox multivariable analysis and risk of treatment discontinuation and serious adverse effects (SAEs) followed an opposite trend [26]. With increasing incidence of MM in elderly population and prevalence of elderly MM survivors on long term oral maintenance therapy with advent of novel agents, larger studies are needed to ascertain basis for biological heterogeneity of MM in different age brackets.
Race
The incidence of MM and its precursor Monoclonal Gammopathy of Undetermined Significance (MGUS) has been shown to be two to three-fold higher in African-Americans (AAs) compared to Caucasians [27]. A study on survival among Randomized Clinical Trial (RCT) patients in Southwest Oncology Group (SWOG) did not show any statistically significant racial disparity among MM patients [28]. Another study found AA patients tend to relapse early after ASCT in MM, mechanism of which was unclear [29]. A large population based study using SEER database, however, showed disease-specific and RS rates to be higher in blacks throughout the study period 1973-2005, but with significant survival improvement in whites compared to blacks in the same time-period [30]. Another SEER-based comparative analysis of ethnic sub-groups showed Asians having best median OS (2.7 years) and myeloma specific survival (4.1 years) and Hispanics with worst median OS (2.4 years) [31].
Socio-economic status (SES)
Small studies with conflicting results have been performed to assess the effect of SES on myeloma survival, with no rigorous large population-based study till date [32,33]. The trend of temporal improvement in survival of Caucasians over time in various largescale population based studies could be related to better SES and access to care [9,31]. With advent of novel agents and rising cost of myeloma therapy [34], SES and access to specialized care might become an important predictor of outcome and will require judicious allocation of resources.
Performance status (PS)
The Eastern Co-operative Oncology Group (ECOG) PS has been shown in a univariate analysis to predict prognosis in MM, with poor PS (3 or 4) being associated with shorter median OS [35]. In a multivariate analysis, ECOG-PS has been shown to significantly predict survival in MM patients with spinal cord compression [36]. PS is a simple and inexpensive tool, which can be readily, assesses at bedside, but involves subjective assessment.
Renal function
In a study on elderly patients enrolled in European phase III trials, the risk of death was found to be increased in patients with renal failure, defined as creatinine level = 2 mg/dl (HR 2.02, 95% CI: 1.51-2.70; P<0.001) [37]. Renal impairment (RI) has been shown to be associated with increased frequency of adverse genetic factors, including del17p or t(4;14) using Fluorescence In Situ Hybridization (FISH) [38]. Bortezomib has been shown to overcome the adverse impact of RI in newly diagnosed MM patients, which could be partly due to its effectiveness in MM patients with high-risk cytogenetics [38]. RI was not found to be independently associated with inferior survival in a study on 203 consecutive MM patients treated upfront with novel agents, including thalidomide, lenalidomide and bortezomib [39]. As RI is a common presentation of MM, being more so in elderly population, novel agents might improve overall prognosis in this subgroup in future.
Tumor-related Factors
β-2 microglobulin (β2M)
Serum β2M is a Major Histocompatibility Complex (MHC) class I subunit, which is renally excreted, used as a surrogate for tumor burden and has been shown in many studies to correlate with survival in patients with MM [40-45]. In ISS, serum β2M level = 5.5 mg/dl is used to classify MM patients as stage III and portends a median survival of 29 months [46]. A study demonstrated β2M uncorrected for stage and serum creatinine level to be the most significant prognostic factor after adjustment for age [41]. If the serum β2M level is corrected for the renal function, its prognostic influence in patients of renal failure is lost. Another study showed a combination of chromosome 13 abnormalities identified by FISH and β2M level to be a strong prognostic factor in MM patients receiving HDT [43]. In MM patients undergoing HDT followed by ASCT, β2M has been shown to predict OS and Progression-Free Survival (PFS) in univariate analysis [44]. Serum β2M, however, is not predictive of long-term survival [47].
Albumin
Serum albumin is an inexpensive laboratory test and provides us with valuable information on prognosis of MM. The combination of serum albumin and β2M has been shown to be the most powerful prognostic factor in ISS [46]. A review on around 1000 patients with newly diagnosed MM had shown serum albumin as an important prognostic factor on multivariate analysis [35]. Agarose gel serum protein electrophoresis (PEL), which is used universally to measure albumin as well as monoclonal (M) protein estimate, has been shown to be equally accurate in predicting survival as bromcresol green (BCG) assay in a study, highlighting the need to eliminate additional testing [48].
C-reactive protein (CRP)
CRP is an acute phase reactant of hepatic origin and increases following cellular secretion of interleukin-6 (IL-6) [49]. CRP and alpha-1-antitrypsin (AAT) has been shown significantly correlate with survival in a univariate analysis [50]. Other studies with conflicting evidence did not find any correlation between IL-6 and survival or tumor burden in MM [51,52]. Given lack of mature data on prognostic significance of CRP, it has not been incorporated in risk stratification models of MM till date.
Lactate dehydrogenase (LDH)
Studies have shown high LDH levels in MM to be associated with high tumor burden, occult extra-osseous disease, poor survivaland overall prognosis [44,53-55]. Interestingly, poor prognosis was also noted in patients with normal LDH levels at presentation but elevated level after high-dose cytotoxic chemotherapy [54]. Study conducted on database of Greek Myeloma Study Group found high LDH to be associated with inferior OS within all ISS groups. In patients receiving novel agents, median OS of high and normal LDH group was 21 and 51 months respectively [56].
Serum free light chain (FLC) assay
The immunoglobulin FLC is an important tool for following patients with oligosecretory MM [57]. FLC provides a rapid assessment of response to therapy, given its short half-life [58]. A study has shown higher 5-year disease specific survival in MM patients with serum FLC ratio (κ/λ or λ/ κ depending on dominating monoclonal light chain) lower than median [59]. Another larger study on 790 newly diagnosed MM patients had similar findings and proposed incorporation of serum FLC ratio into ISS for better risk stratification [60]. However, serum FLC levels in MM patients treated with alkylator based therapy did not seem to correlate with 24-hour urine protein level, questioning its significance in followup of patients having M-protein measurable by electrophoresis [57]. A study has shown serum FLC ratio at the time of stem cell mobilization to have prognostic significance on survival endpoints following novel agent-based induction therapy and autologous SCT [61]. International Myeloma Working group (IMWG) has added normal serum FLC ratio as a criteria for stringent complete response in MM [60].
Complete blood count (CBC)
A study has shown anemia in over 70% of MM patients on initial presentation [35]. Although hemoglobin level is included in Durie- Salmon staging system, it was dropped in ISS due to lack of prognostic significance upon multivariate analysis [46]. Anemia has been found to be associated with RI in a study on newly diagnosed MM [39]. Thrombocytopenia has been identified as an important prognostic factor on multivariate analysis in a study [35]. Although platelet count ranked highly in prognostic significance in a study, it was present only in 12% of patients, limiting its widespread use [46]. However, platelets count <130,000/μL was found to identify patient subset with very poor prognosis, with a hazard ratio (HR) of 1.63 on multivariate analysis, ranking next to serum β2M. Absolute Lymphocyte Count (ALC) has been identified as an independent prognostic factor for OS in newly diagnosed MM patients [62]. Early ALC recovery has been shown to predict both OS and PFS in MM patients after SCT [63]. Ratio of ALC to Absolute Monocyte Count (AMC) was found to be an independent prognostic factor for OS in a Korean study, with low ALC/AMC ratio, being associated with high ISS stage [64]. CBC is an inexpensive test and can provide an incredible wealth of indirect information on prognosis of MM.
Bone marrow morphology
Bone marrow aspiration and biopsy is required for diagnosis of MM [2]. A study has shown plasmablastic morphology to be associated with poor response rate, aggressive disease and shortened survival [65]. The plasmablastic cases in this study were found to have lower albumin levels, higher β2M levels and higher percentage of bone marrow plasma cells by immunofluorescence. However, morphologic features suggested by Bartl et al. [66] have found limited space in risk stratification due to variable distribution of plasma cells in marrow.
Cytogenetics
Cytogenetics is an essential part of initial workup for risk stratification in MM and can be performed by conventional methods, or, more commonly by techniques not based on metaphase availability, including FISH, array comparative genomic hybridization and Single Nucleotide Polymorphism (SNP) based mapping arrays [67]. Among karyotypic abnormalities, hyperdiploidy is present in about half of newly diagnosed MM patients and is usually associated with a favorable prognosis [68,69]. Hyperdiploidy usually co-exists with Immunoglobulin Heavy chain (IgH) chromosomal translocations, primarily involving chromosome 14 and has been shown in a study to even precede IgH translocation in a proportion of patients [68,70]. Non-hyperdiploidy, on the other hand, is associated with poor OS, PFS and Event Free Survival (EFS) [69,71].
Patients with t(4;14) and t(14;16) have a dismal prognosis, compared to patients with t(11;14) [72]. The oncogenes involved in these translocations include Fibroblast Growth Factor-3 (FGFR-3), Multiple Myeloma SET Domain (MMSET) and c-MAF [72]. Newly diagnosed MM patients with t(4;14) have been shown to have poor survival in the setting of conventional therapy, HDT and ASCT [69,71,73]. There has been conflicting evidence on the effect of bortezomib on survival in patients with t(4;14) translocation[74,75]. Long-term follow-up of 100 MM patients with t(4;14) treated with tandem transplantation identified a sub-group of patients with low β2M and high hemoglobin level to have superior median OS and EFS [76], indicating its heterogeneity in MM patient population. A recent study on 1003 MM patients did not find any adverse prognostic significance of t(14;16) on multivariate analysis [77], contrary to prior studies [72,78]. Gain of chromosome 1 (+1q21) and t(14;20) has also been shown to define adverse prognosis in the context of thalidomide and conventional induction therapy, with or without ASCT [78].
Deletions of chromosome 17p and 13q are seen commonly in MM and portends a poor prognosis [72]. Chromosome 17 harbors tumor suppressor gene p53 in locus 17p13.1, deletion of which has been shown to be associated with shorter OS and PFS in MM patients undergoing HDT followed by ASCT [79]. Combining del(17p) status obtained by FISH with ISS staging has been shown to improve prognostic assessment in various studies [69,71,78,80]. Short-term induction therapy with novel agent bortezomib did not improve outcome of patients with del(17p) [74] but therapy with bortezomibbased regimen both before and after ASCT was found to reduce the adverse impact of del(17p) on PFS and OS [81]. del(17p) was not found to be an independent adverse prognostic factor in patients with Gene Expression Profiling (GEP) derived low-risk disease receiving bortezomib containing Total Therapy 3 (TT3) [82]. Monosomy and/ or deletions of chromosome 13 (Δ13) has poor prognostic effect on MM patients treated with HDT [83]. Clinically, it leads to deletion of Rb gene, which is associated with poor PS, high creatinine, CRP and LDH levels, high percentage of BM plasma cells and advanced stage. A study has shown deletion of 13q14 identified by interphase FISH (iFISH) to be associated with increased proliferative activity (Ki-67) and shortened survival [84]. In a study comparing detection of Δ13 by conventional cytogenetics versus iFISH, there was no prognostic significance of abnormalities detected by iFISH alone, indicating metaphase analysis by conventional cytogenetics to be a preferred approach for testing Δ13 [85]. Bortezomib has been shown to overcome poor prognosis due to Δ13 in many studies [86-88]. Δ13 detected by conventional cytogenetics has been included in classification of intermediate risk active MM by Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines [4].
Gene expression profiling (GEP)
To identify molecular basis of MM at a transcriptional level, Zhan et al. performed mRNA expression profiling in CD-138 positive plasma cells from MM patients undergoing HDT and tandem SCTs [89]. They introduced 7 molecular subtypes of MM: PR (proliferation), LB (low bone disease), MS (MMSET), HY (hyperdiploid), CD-1 (CCND1), CD-2 (CCND3) and MF (MAF/MAFB). PR and MS groups were associated with significant over-expression of 1q genes and poor survival compared with other groups. When this model was applied to relapsed MM patients enrolled in APEX phase 3 trials treated with single-agent bortezomib or high-dose dexamethasone, OS estimates of 1-year actuarial probabilities were 74% for lowrisk disease versus 32% for high-risk disease [90]. Another study on newly diagnosed MM patients identified 3 additional subsets, over-expressing genes involved in Nuclear Factor (NF) kappa lightchain- enhancer (TNFAIP3 and CD40), cancer testis antigens and protein tyrosine phosphatases (PRL-3, PTPRZ1 and SOCS3) [91]. A 92-gene prognostic index (EMC-92 gene signature) was developed by GEP and validated in newly diagnosed as well as relapsed MM patients included in HOVON65/GMMG-HD4 trial [92]. Patients defined high-risk by EMC-92 gene signature had reduced OS and it remained an independent prognostic factor on multivariate analysis. In a study of Intergroupe Francophone du Myélome (IFM), patients were stratified into high and low risk based on expression of 15 genes involved in cell cycle and chromosomal stability. High-risk patients, with 3-year survival rate of 47.4%, were characterized by over-expression of genes involved in cell cycle progression. Low-risk patients, with 3-year survival rate of 90.5%, displayed hyperdiploidy and heterogeneity in genetic signatures. GEP is a valuable tool but needs further validation in larger patient populations before incorporation into general practice and risk stratification models.
Immunophenotyping
Myelomatous plasma cells (PCs) have been shown to express additional cell surface markers compared to normal BM PCs, which warrants further investigation into their prognostic and therapeutic significance [93]. Over-expression of CD28 by myeloma cells have been shown to be associated with tumoral expansion and treatment failure [94]. Absence of some cell surface markers, like CD45 and CD56 have been associated with plasma cell leukemia (PCL) and an aggressive subset of MM [95,96]. PCs lacking or weakly expressing CD56 have low osteolytic potential and tend to disseminate in peripheral blood. MM patients with CD45 negative PCs were shown to have worse OS on multivariate analysis [96]. Similarly, CD27 negative MM patients had worse 3-year OS in a study (50% versus 92% in CD 27 positive patients) [97]. CD 117 (c-kit), which is not expressed in normal PCs was found in 50% of MGUS, 33 % of newly diagnosed MM and 8% of relapsed MM patients [98]. However, CD117 expression was associated with better 4-year OS. CD117 negative patients with poor prognosis expressed CD221 and had t(4;14) in this study. In a prospective study on prognostic impact of immunophenotypic markers in newly diagnosed MM patients treated per GEM 2000 protocol, expression of CD19 and CD28 and lack of expression of CD117 was associated with significantly shorter OS and PFS [99]. This study proposed a risk stratification model based on simultaneous assessment of CD28 and CD117: Poor risk (CD28 positive CD117 negative), Intermediate risk (both markers negative and positive), and Good risk (CD28 negative CD117 positive). CD28 expression was associated with t(14;16) and del(17p). A splice variant of CD44, CD44v6 was found to be associated with 13q14 deletion and expressed in 43% of ISS stage II/III MM or PCL patients in a study [100]. Further immunophenotypic studies are required in large phase III trials of MM prior to treatment initiation to assess correlation with outcomes and association with critical cytogenetic abnormalities [101].
Proliferative capacity
Plasma Cell Labelling Index (PCLI) is a surrogate for proliferative capacity of tumor cells in MM and has been shown to be an independent prognostic factor in numerous studies [44,102-105]. MM patients with high PCLI in stable plateau phase after induction regimen were found to have shorter median Time To Progression (TTP) and OS compared to high PCLI group (8 and 20 months in low PCLI versus 39 and 56 months in high PCLI groups respectively) [103]. A significant reduction in PCLI after initiation of therapy in newly diagnosed MM was an important predictor of survival, independent of β2M, creatinine, serum M-spike response and baseline PCLI in a study [104]. Another study on 595 patients with MM undergoing HDT/ASCT in the Spanish GEM2000 and GEM2005 <65y trials found high PCLI (defined as =1% PCs in S-phase) assessed by multiparameter flow cytometry as an independent prognostic factor of OS [44]. However, the inferior OS was overcome by treatment with bortezomib based regimens. GEP-based proliferation assessment has also been shown to be an independent prognostic factor for EFS and OS, with higher proliferation indices being associated with +1q21 and del(13q14) and lower with hyperdiploid signatures [105]. Ki-67 is a nuclear protein expressed by dividing cells and can be used to identify proliferating cells in G1, G2, S and M phase of cell-cycle [67]. A study on Ki-67 proliferation index found its expression to increase with increasing Durie-Salmon stage and better survival in patients with Ki-67 expression less than 8% [45]. Proliferation of malignant PCs in MM, as assessed by various techniques discussed above, can be used as an important adjunct to current risk stratification models for defining prognosis and also be targeted by novel compounds in future, including tubulin polymerase and aurora kinase inhibitors [106,107].
Imaging
Although conventional radiographs have remained a cornerstone of initial diagnostic workup in MM, advanced imaging including Computerized Tomography (CT), Magnetic Resonance Imaging (MRI), Positron Emission Tomography (PET) and (99) Technetium sestamibi (MIBI) have increasingly been used for diagnostic workup, prognostication and assessing response to therapy[108].
Magnetic Resonance Imaging (MRI) is useful for detecting diffuse and focal bone marrow infiltration in the absence of osteolysis on conventional radiographs in Metastatic Bone Surveys (MBS) [109]. Various MRI patterns of the BM infiltration-normal, focal, diffuse and variegated - have been used to predict OS [108]. A study on around 600 MM patients treated with tandem ASCT showed identification of MRI-based Focal Lesions (FL) to be an independent predictor of survival [109]. A risk stratification model was created based on presence of cytogenetic abnormalities and more than 7 FL on MRI: 5-year survival was 76% in the absence of both, 61% in the presence of 1 FL on MRI and 37% in the presence of both. FL identified by MBS did not have any prognostic significance. Another study showed focal and diffuse infiltration patterns on whole body MRI after ASCT to correlate with OS [110]. Diffuse marrow infiltration on MRI portends a poor prognosis and has been shown to further stratify previously untreated MM patients with ISS stages I and II [111].
A study on 13 patients with MM showed F-18 Fluorodeoxyglucose (FDG) PET to be able to detect medullary involvement, residual or recurrent tumor, post-therapeutic changes and response to therapy in MM [112]. Sensitivity of FDG-PET in detecting Myelomatous changes was 85% and specificity was 92% in this study. Another study tested PET/CT in newly diagnosed MM patients treated with thalidomide-based induction therapy followed by double ASCT [113]. Standardized Uptake Value (SUV) > 4.2 and Extra-Medullary Disease (EMD) at baseline was associated with shorter 4-year OS. Persistence of FDG uptake after ASCT, SUV > 4.2 and EMD were independent predictors of poor PFS on multivariate analysis. Number of FDG-avid FLs on PET/CT has been shown to correlate with high β2M, CRP and LDH levels [114]. In MM patients treated with Total Therapy 3 (TT3), presence of >3 FLs on day 7 of induction therapy was indicative of inferior OS and PFS, overall and also in patients with GEP-defined high-risk disease [115]. A prospective study comparing imaging modalities in MM found MRI of spine and pelvis to be superior technique for detecting marrow involvement, but PET/ CT enabled detection of myelomatous lesions in areas out of the field of view of MRI [116].
Response to Therapy
The impact of Complete Response (CR), defined as disappearance of M-protein on immunofixation, on survival of MM has been controversial, with conflicting evidences in literature. CR has been shown to be a prognostic indicator of long-term PFS and OS in patients treated by HDT/ASCT, Total Therapy (TT) protocols and novel agents [117-121]. A study on 1175 elderly MM patients treated with novel agents showed CR to be an independent predictor of longer PFS and OS irrespective of age, ISS stage and treatment [117]. Another study on long-term survivors of MM after ASCT found CR to be an independent prognostic factor, with 12-year OS being 35% in patients who achieved CR, compared to 22%, 16% and 16% in patients achieving near CR (nCR), Very Good Partial Response (VGPR) and Partial Response (PR) respectively [120]. However, the differential significance of CR as a prognostic factor was studied in GEP-based subgroups of patients, which revealed its significance only in very high-risk subgroup [121]. A study on MM patients enrolled in SWOG phase III trials showed TTP, but not the magnitude of response, to be an independent predictor of survival [122].
In a study on relapsed MM patients after ASCT, median OS from relapse was significantly shorter in patients who had early relapse (ER), =12 months from ASCT (10.8 months in ER group versus 41.8 for rest, p<0.0001) [123]. Failure to achieve CR, along with PCLI =1% and greater than 1 treatment regimen prior to ASCT was shown in this study to independently predict early relapse on multivariate analysis. Median OS from diagnosis and from ASCT was significantly shorter in ER group as well. A Canadian study showed ER to be an independent adverse prognostic factor for OS in MM patients receiving ASCT with novel agent based induction regimen [124].
Minimal residual disease (MRD) has been shown to be have prognostic significance after therapy for MM and persistent MRD detected by multiparameter-flow-cytometry (MFC), polymerasechain- reaction (PCR), next generation sequencing (NGS) and PET/ CT has been shown to confer poor survival among patients who achieve CR [125]. In a multicenter randomized phase 3 trial, each log depletion of MRD was associated with significant improvement of OS (median OS of 1, 4, 5.9, 6.8 and >7.5 years for MRD =10%, <10%, 0.1% to <1%, 0.01% to <0.1% and <0.01% respectively) [126].
Clinical Application
Several risk stratification models have been developed for prognosticating patients with MM, of which, few are used widely in clinical practice worldwide.
ISS
ISS was developed after analysis of clinical and laboratory features of more than 10,000 previously untreated symptomatic MM patients from North America, Europe and Asia before 2002 [46]. It is fairly simple to understand and reproduce in daily clinical practice. Based on serum albumin and β2M level, its stratifies patients into three stages: Stage I, β2M less than 3.5 mg/L plus serum albumin =3.5 g/dL (median survival, 62 months); Stage II, neither stage I nor III (median survival, 44 months); and Stage III, β2M = 5.5 mg/L (median survival, 29 months). ISS staging was validated in patients receiving both conventional therapy as well as ASCT in all age groups. However, the validity of ISS in the era of novel therapeutic agents has been questioned by some studies [127,128]. An analysis of Greek Myeloma Study Group (GMSG) found ISS to be valid in patients receiving front-line therapy with novel agents (4-year OS was 85, 61 and 26% for ISS stage I, II and III patients, P=0.001) [127]. However, another European study found that ISS had no significant impact on survival of newly diagnosed MM patients receiving therapy with novel agents [128].
mSMART
mSMART is a risk stratification model based on consensus recommendations from Mayo Clinic myeloma physicians and uses a combination of conventional metaphase cytogenetics, PCLI, FISH and GEP [4]. It stratifies patients into three groups: High risk, with del(17p), t(14;16), t(14;20) and high risk GEP signature; Intermediate risk, with t(4;14), del(13), hypodiploidy and PCLI = 3% and Standard risk, which includes t(11;14), t(6;14) among others. The median OS in years is 3, 4-5 and 8-10 in high, intermediate and standard risk patients respectively. Bortezomib based regimes are recommended for patients with t(4; 14) For high risk patients, this model advocates use of bortezomib-lenalidomide-dexamethasone as induction therapy before ASCT, based on current evidence.
IMWG
IMWG advocates use of ISS staging in conjunction with FISH for t(4; 14), deletion 17p13 and 1q21 gain for risk stratification in MM [129]. High risk group is defined as ISS stage II/III and the presence of either t(4; 14) or 17p13 and low risk group is defined as age < 55 years, ISS stage I/II and normal results for the three FISH markers. The median OS in high, standard and low risk patients was 2, 7 and >10 years respectively in this model. Testing for gain of 1q or del(1p) has been advocated by European Myeloma Network (EMN) in addition to above-mentioned cytogenetic abnormalities [130].
Intergroupe francophone du myélome
Data from Intergroupe Francophone du Myélome (IFM) trials were used to create a prognostic model to evaluate risk of death related to MM progression within 2 years of treatment initiation [131]. Three independent prognostic variables identified including LDH, ISS III and adverse cytogenetics [t(4;14) and/or del(17p)], were used to create a score ranging from 0-3. The odds ratio estimates for MM progression-related death were 1, 2.9, 5.7 and 24.0 for scores of 0, 1, 2 and 3 respectively. This prognostic index was validated in three other European trials and was found to segregate patients receiving bortezomib-based induction therapy in these trials into four categories based on scores 0-3.
A comparison of new risk stratification models, including mSMART, IMWG and IFM have been presented in Table 1.
Prognostic model
Variables
Risk categories
Median OS/ Risk of progression-related death
Reference
mSMART
Cytogenetics (FISH), GEP and PCLI
High risk: del(17p), t(14;16) and t(14;20) on FISH and high-risk signature on GEP
Intermediate risk: t(4;14) on FISH, cytogenetic deletion 13, hypodiploidy and PCLI =3%
Standard risk: All others including t(11;14) and t(6;14) on FISH
Median OS: 3, 4-5 and 8-10 years in high, intermediate and standard risk respectively
[4]
IMWF
ISS (I/II/III) and cytogenetics (FISH)
High risk: ISS II/III and t(4;14) or 17p13 deletion
Low risk: ISS I/II and absence of t(4;14), 17p13 deletion and +1q21 and age <55 years
Standard risk: Others
Median OS 2, 7 and >10 years in high, standard and low-risk respectively
[128]
IFM
LDH, ISS (III) and cytogenetics (FISH)
Scores 0-3 (higher score indicating poor prognostic subgroup), with 1 point each for high LDH, ISS III and adverse cytogenetics [t(4;14) and/or del (17p)]
Odds ratio estimate for MM progression-related death 2 years from treatment initiation: 1, 2.9, 5.7 and 24.0 for scores 0, 1, 2 and 3 respectively
[130]
Table 1: Overview of novel risk stratification models for multiple myeloma.
Conclusion
As biological heterogeneity of MM is increasingly being recognized, cytogenetics and molecular abnormalities with be used widely for risk stratification in future [129]. High response of novel agents in patients with high-risk cytogenetics has led to increased acceptance of risk-adapted therapy and improved survival in recent decades. A study on prospective analysis of GEP in CD138 positive PCs is currently ongoing for classification and risk stratification of MM (NCT01619358). Another study on identifying molecular characteristics of MM by FISH, SNP, GEP and mRNA expression profiling is being conducted with secondary outcome of predicting OS and EFS (NCT00639054). Further studies are required to unravel cytogenetic correlation of GEP and immunophenotypic signatures and development of new models which are prospectively tested in the context of novel agents.
References
- Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008; 111: 2962-2972.
- Vincent Rajkumar S. Multiple myeloma: 2014 Update on diagnosis, risk-stratification, and management. Am J Hematol. 2014; 89: 999-1009.
- Solly S. Remarks on the pathology of mollities ossium; with cases. Med Chir Trans. 1844; 27: 435-498.
- Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013; 88: 360-376.
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64: 9-29.
- Moreau P, San Miguel J, Ludwig H, Schouten H, Mohty M, Dimopoulos M, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology. 2013; 24: vi133-137.
- Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ 3rd, et al. Incidence of multiple myeloma in Olmsted County, Minnesota: Trend over 6 decades. Cancer. 2004; 101: 2667-2674.
- Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J Clin Oncol. 2010; 28: 830-834.
- Manikkam Umakanthan J, Uprety D, Kasireddy V. Analyzing Survival Trends in Multiple Myeloma Patients in Pre and Post-Bortezomib Era Using the SEER Database. 2014; 124.
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008; 111: 2516-2520.
- Badros AZ. In the age of novel therapies, what defines high-risk multiple myeloma? J Natl Compr Canc Netw. 2010; 8 Suppl 1: S28-34.
- Dewald GW, Therneau T, Larson D, Lee YK, Fink S, Smoley S, et al. Relationship of patient survival and chromosome anomalies detected in metaphase and/or interphase cells at diagnosis of myeloma. Blood. 2005; 106: 3553-3558.
- Bergsagel PL. Prognostic factors in multiple myeloma: it's in the genes. Clin Cancer Res. 2003; 9: 533-534.
- Lonial S. Designing risk-adapted therapy for multiple myeloma: the Mayo perspective. Mayo Clin Proc. 2007; 82: 279-281.
- Fonseca R, San Miguel J. Prognostic factors and staging in multiple myeloma. Hematol Oncol Clin North Am. 2007; 21: 1115-1140, ix.
- San-Miguel J, Harousseau JL, Joshua D, Anderson KC. Individualizing treatment of patients with myeloma in the era of novel agents. J Clin Oncol. 2008; 26: 2761-2766.
- Mileshkin L, Prince HM. The adverse prognostic impact of advanced age in multiple myeloma. Leuk Lymphoma. 2005; 46: 951-966.
- Ludwig H, Durie BG, Bolejack V, Turesson I, Kyle RA, Blade J, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008; 111: 4039-4047.
- Chretien ML, Hebraud B, Cances-Lauwers V, Hulin C, Marit G, Leleu X, et al. Age is a prognostic factor even among patients with multiple myeloma younger than 66 years treated with high-dose melphalan: the IFM experience on 2316 patients. Haematologica. 2014; 99: 1236-1238.
- Lenhoff S, Hjorth M, Holmberg E, Turesson I, Westin J, Nielsen JL, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood. 2000; 95: 7-11.
- Corso A, Klersy C, Lazzarino M, Bernasconi C. Multiple myeloma in younger patients: the role of age as prognostic factor. Ann Hematol. 1998; 76: 67-72.
- García-Sanz R, González-Fraile MI, Mateo G, Hernández JM, López-Berges MC, de las Heras N, et al. Proliferative activity of plasma cells is the most relevant prognostic factor in elderly multiple myeloma patients. Int J Cancer. 2004; 112: 884-889.
- Nilsson T, Lenhoff S, Turesson I, Rylander L, Mitelman F, Westin J, et al. Cytogenetic features of multiple myeloma: impact of gender, age, disease phase, culture time, and cytokine stimulation. Eur J Haematol. 2002; 68: 345-353.
- Sagaster V, Kaufmann H, Odelga V, Ackermann J, Gisslinger H, Rabitsch W, et al. Chromosomal abnormalities of young multiple myeloma patients (<45 yr) are not different from those of other age groups and are independent of stage according to the International Staging System. Eur J Haematol. 2007; 78: 227-234.
- Siegel DS, Desikan KR, Mehta J, Singhal S, Fassas A, Munshi N, et al. Age is not a prognostic variable with autotransplants for multiple myeloma. Blood. 1999; 93: 51-54.
- Bringhen S, Evangelista A, Offidani M, Ballanti S, Zaccaria A, Pescosta N, et al. A Simple Score, Based On Geriatric Assessment, Improves Prediction of Survival, and Risk Of Serious Adverse Events In Elderly Newly Diagnosed Multiple Myeloma Patients. 2013; 122.
- Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia. 2009; 23: 1691-1697.
- Albain KS, Unger JM, Crowley JJ, Coltman CA Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. Je Natl Cancer Inst. 2009; 101: 984-992.https://www.ncbi.nlm.nih.gov/pubmed/19139731
- Khaled Y, Abidi MH, Janakiraman N, Kato K, Levine JE, Reddy P, et al. Outcomes after auto-SCT in African Americans with multiple myeloma. Bone Marrow Transplant. 2009; 43: 845-851.
- Waxman AJ, Mink PJ, Devesa SS, Anderson WF, Weiss BM, Kristinsson SY, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood. 2010; 116: 5501-5506.
- Ailawadhi S, Aldoss IT, Yang D, Razavi P, Cozen W, Sher T, et al. Outcome disparities in multiple myeloma: a SEER-based comparative analysis of ethnic subgroups. Br J Haematol. 2012; 158: 91-98.
- Weston B, Grufferman S, MacMillan JP, Cohen HJ. Effects of socioeconomic and clinical factors on survival in multiple myeloma. J Clin Oncol. 1987; 5: 1977-1984.
- Pasqualetti P, Colantonio D, Collacciani A, Casale R. [Socioeconomic status and survival in multiple myeloma]. Minerva Med. 1990; 81: 713-716.
- Gaultney JG, Uyl-de Groot CA. Efficient allocation of novel agents in multiple myeloma: a work in progress. Oncologist. 2013; 18: 5-7.
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78: 21-33.
- Rades D, Douglas S, Veninga T, Poortmans P, Bajrovic A, Hoskin PJ, et al. Prognostic factors for local control and survival in patients with spinal cord compression from myeloma. Strahlenther Onkol. 2012; 188: 628-631.
- Bringhen S, Mateos MV, Zweegman S, Larocca A, Falcone AP, Oriol A, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013; 98: 980-987.
- Scheid C, Sonneveld P, Schmidt-Wolf IG, van der Holt B, el Jarari L, Bertsch U, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014; 99: 148-154.
- Eleftherakis-Papapiakovou E, Kastritis E, Roussou M, Gkotzamanidou M, Grapsa I, Psimenou E, et al. Renal impairment is not an independent adverse prognostic factor in patients with multiple myeloma treated upfront with novel agent-based regimens. Leuk Lymphoma. 2011; 52: 2299-2303.
- Bataille R, Boccadoro M, Klein B, Durie B, Pileri A. C-reactive protein and beta-2 microglobulin produce a simple and powerful myeloma staging system. Blood. 1992; 80: 733-737.
- Greipp PR, Katzmann JA, O'Fallon WM, Kyle RA. Value of beta 2-microglobulin level and plasma cell labeling indices as prognostic factors in patients with newly diagnosed myeloma. Blood. 1988; 72: 219-223.
- Bataille R, Durie BG, Grenier J. Serum beta2 microglobulin and survival duration in multiple myeloma: a simple reliable marker for staging. Br J Haematol. 1983; 55: 439-447.
- Facon T, Avet-Loiseau H, Guillerm G, Moreau P, Geneviève F, Zandecki M, et al. Chromosome 13 abnormalities identified by FISH analysis and serum beta2-microglobulin produce a powerful myeloma staging system for patients receiving high-dose therapy. Blood. 2001; 97: 1566-1571.
- Paiva B, Vídriales MB, Montalbán MÁ, Pérez JJ, Gutiérrez NC, Rosiñol L, et al. Multiparameter flow cytometry evaluation of plasma cell DNA content and proliferation in 595 transplant-eligible patients with myeloma included in the Spanish GEM2000 and GEM2005<65y trials. Am J Pathol. 2012; 181: 1870-1878.
- Alexandrakis MG, Passam FH, Kyriakou DS, Dambaki K, Niniraki M, Stathopoulos E, et al. Ki-67 proliferation index: correlation with prognostic parameters and outcome in multiple myeloma. Am J Clin Oncol. 2004; 27: 8-13.
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005; 23: 3412-3420.
- Kyle RA. Why better prognostic factors for multiple myeloma are needed. Blood. 1994; 83: 1713-1716.
- Kapoor P, Snozek CL, Colby C, Larson DR, Katzmann JA, Rajkumar SV, et al. Clinical impact of discordance in serum albumin measurements on myeloma international staging system. J Clin Oncol. 2008; 26: 4051-4052.
- Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999; 7: 169-177.
- Merlini G, Perfetti V, Gobbi PG, Quaglini S, Franciotta DM, Marinone G, et al. Acute phase proteins and prognosis in multiple myeloma. Br J Haematol. 1993; 83: 595-601.
- Ballester OF, Moscinski LC, Lyman GH, Chaney JV, Saba HI, Spiers AS, et al. High levels of interleukin-6 are associated with low tumor burden and low growth fraction in multiple myeloma. Blood. 1994; 83: 1903-1908.
- Ohtani K, Ninomiya H, Hasegawa Y, Kobayashi T, Kojima H, Nagasawa T, et al. Clinical significance of elevated soluble interleukin-6 receptor levels in the sera of patients with plasma cell dyscrasias. Br J Haematol. 1995; 91: 116-120.
- Dimopoulos MA, Barlogie B, Smith TL, Alexanian R. High serum lactate dehydrogenase level as a marker for drug resistance and short survival in multiple myeloma. Ann Intern Med. 1991; 115: 931-935.
- Barlogie B, Smallwood L, Smith T, Alexanian R. High serum levels of lactic dehydrogenase identify a high-grade lymphoma-like myeloma. Ann Intern Med. 1989; 110: 521-525.
- Suguro M, Kanda Y, Yamamoto R, Chizuka A, Hamaki T, Matsuyama T, et al. High serum lactate dehydrogenase level predicts short survival after vincristine-doxorubicin-dexamethasone (VAD) salvage for refractory multiple myeloma. Am J Hematol. 2000; 65: 132-135.
- Terpos E, Katodritou E, Roussou M, Pouli A, Michalis E, Delimpasi S, et al. High serum lactate dehydrogenase adds prognostic value to the international myeloma staging system even in the era of novel agents. Eur J Haematol. 2010; 85: 114-119.
- Dispenzieri A, Zhang L, Katzmann JA, Snyder M, Blood E, Degoey R. Appraisal of immunoglobulin free light chain as a marker of response. Blood. 2008; 111: 4908-4915.
- Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR, et al. Serum free light chains for monitoring multiple myeloma. Br J Haematol. 2004; 126: 348-354.
- Kyrtsonis MC, Vassilakopoulos TP, Kafasi N, Sachanas S, Tzenou T, Papadogiannis A, et al. Prognostic value of serum free light chain ratio at diagnosis in multiple myeloma. Br J Haematol. 2007; 137: 240-243.
- Snozek CL, Katzmann JA, Kyle RA, Dispenzieri A, Larson DR, Therneau TM, et al. Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: proposed incorporation into the international staging system. Leukemia. 2008; 22: 1933-1937.
- Mori S, Crawford BS, Roddy JV, Phillips G, Elder P, Hofmeister CC, et al. Serum free light chains in myeloma patients with an intact M protein by immunofixation: potential roles for response assessment and prognosis during induction therapy with novel agents. Hematol Oncol. 2012; 30: 156-162.
- Ege H, Gertz MA, Markovic SN, Lacy MQ, Dispenzieri A, Hayman SR, et al. Prediction of survival using absolute lymphocyte count for newly diagnosed patients with multiple myeloma: a retrospective study. Br J Haematol. 2008; 141: 792-798.
- Kim H, Sohn HJ, Kim S, Lee JS, Kim WK, Suh C, et al. Early lymphocyte recovery predicts longer survival after autologous peripheral blood stem cell transplantation in multiple myeloma. Bone Marrow Transplant. 2006; 37: 1037-1042.
- Shin SJ, Roh J, Kim M, Jung MJ, Koh YW, Park CS, et al. Prognostic significance of absolute lymphocyte count/absolute monocyte count ratio at diagnosis in patients with multiple myeloma. Korean J Pathol. 2013; 47: 526-533.
- Greipp PR, Leong T, Bennett JM, Gaillard JP, Klein B, Stewart JA, et al. Plasmablastic morphology--an independent prognostic factor with clinical and laboratory correlates: Eastern Cooperative Oncology Group (ECOG) myeloma trial E9486 report by the ECOG Myeloma Laboratory Group. Blood. 1998; 91: 2501-2507.
- Bartl R, Frisch B. Clinical significance of bone marrow biopsy and plasma cell morphology in MM and MGUS. Pathol Biol (Paris). 1999; 47: 158-168.
- van de Donk NW, Sonneveld P. Diagnosis and risk stratification in multiple myeloma. Hematol Oncol Clin North Am. 2014; 28: 791-813.
- Fonseca R, Barlogie B, Bataille R, Bastard C, Bergsagel PL, Chesi M, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004; 64: 1546-1558.
- Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myélome. Blood. 2007; 109: 3489-3495.
- Pawlyn C, Melchor L, Murison A, Wardell CP, Brioli A, Boyle EM, et al. Coexistent hyperdiploidy does not abrogate poor prognosis in myeloma with adverse cytogenetics and may precede IGH translocations. Blood. 2015; 125: 831-840.
- Neben K, Jauch A, Bertsch U, Heiss C, Hielscher T, Seckinger A, et al. Combining information regarding chromosomal aberrations t(4;14) and del(17p13) with the International Staging System classification allows stratification of myeloma patients undergoing autologous stem cell transplantation. Haematologica. 2010; 95: 1150-1157.
- Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer. 2002; 2: 175-187.
- Gertz MA, Lacy MQ, Dispenzieri A, Greipp PR, Litzow MR, Henderson KJ, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and -17p13 in myeloma patients treated with high-dose therapy. Blood. 2005; 106: 2837-2840.
- Avet-Loiseau H, Leleu X, Roussel M, Moreau P, Guerin-Charbonnel C, Caillot D, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol. 2010; 28: 4630-4634.
- Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012; 30: 2946-2955.
- Moreau P, Attal M, Garban F, Hulin C, Facon T, Marit G, et al. Heterogeneity of t(4;14) in multiple myeloma. Long-term follow-up of 100 cases treated with tandem transplantation in IFM99 trials. Leukemia. 2007; 21: 2020-2024.
- Avet-Loiseau H, Malard F, Campion L, Magrangeas F, Sebban C, Lioure B, et al. Translocation t(14;16) and multiple myeloma: is it really an independent prognostic factor? Blood. 2011; 117: 2009-2011.
- Boyd KD, Ross FM, Chiecchio L, Dagrada GP, Konn ZJ, Tapper WJ, et al. A novel prognostic model in myeloma based on co-segregating adverse FISH lesions and the ISS: analysis of patients treated in the MRC Myeloma IX trial. Leukemia. 2012; 26: 349-355.
- Chang H, Qi C, Yi QL, Reece D, Stewart AK. p53 gene deletion detected by fluorescence in situ hybridization is an adverse prognostic factor for patients with multiple myeloma following autologous stem cell transplantation. Blood. 2005; 105: 358-360.
- Avet-Loiseau H, Durie BG, Cavo M, Attal M, Gutierrez N, Haessler J, et al. Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: an International Myeloma Working Group collaborative project. Leukemia. 2013; 27: 711-717.
- Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012; 119: 940-948.
- Shaughnessy JD, Zhou Y, Haessler J, van Rhee F, Anaissie E, Nair B, et al. TP53 deletion is not an adverse feature in multiple myeloma treated with total therapy 3. Br J Haematol. 2009; 147: 347-351.
- Perez-Simon JA, Garcia-Sanz R, Tabernero MD, Almeida J, Gonzalez M, Fernandez-Calvo J, et al. Prognostic value of numerical chromosome aberrations in multiple myeloma: A FISH analysis of 15 different chromosomes. Blood. 1998; 91: 3366-3371.
- Zojer N, Königsberg R, Ackermann J, Fritz E, Dallinger S, Krömer E, et al. Deletion of 13q14 remains an independent adverse prognostic variable in multiple myeloma despite its frequent detection by interphase fluorescence in situ hybridization. Blood. 2000; 95: 1925-1930.
- Chiecchio L, Protheroe RK, Ibrahim AH, Cheung KL, Rudduck C, Dagrada GP, et al. Deletion of chromosome 13 detected by conventional cytogenetics is a critical prognostic factor in myeloma. Leukemia. 2006; 20: 1610-1617.
- Jagannath S, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2007; 21: 151-157.
- Mateos MV, Oriol A, Martínez-López J, Gutiérrez N, Teruel AI, de Paz R, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010; 11: 934-941.
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008; 359: 906-917.
- Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, et al. The molecular classification of multiple myeloma. Blood. 2006; 108: 2020-2028.
- Zhan F, Barlogie B, Mulligan G, Shaughnessy JD Jr, Bryant B. High-risk myeloma: a gene expression based risk-stratification model for newly diagnosed multiple myeloma treated with high-dose therapy is predictive of outcome in relapsed disease treated with single-agent bortezomib or high-dose dexamethasone. Blood. 2008; 111: 968-969.
- Broyl A, Hose D, Lokhorst H, de Knegt Y, Peeters J, Jauch A, et al. Gene expression profiling for molecular classification of multiple myeloma in newly diagnosed patients. Blood. 2010; 116: 2543-2553.
- Kuiper R, Broyl A, de Knegt Y, van Vliet MH, van Beers EH, van der Holt B, et al. A gene expression signature for high-risk multiple myeloma. Leukemia. 2012; 26: 2406-2413.
- Ruiz-Argüelles GJ, San Miguel JF. Cell surface markers in multiple myeloma. Mayo Clin Proc. 1994; 69: 684-690.
- Robillard N, Jego G, Pellat-Deceunynck C, Pineau D, Puthier D, Mellerin MP, et al. CD28, a marker associated with tumoral expansion in multiple myeloma. Clin Cancer Research. 1998; 4: 1521-1526.
- Pellat-Deceunynck C, Barillé S, Jego G, Puthier D, Robillard N, Pineau D, et al. The absence of CD56 (NCAM) on malignant plasma cells is a hallmark of plasma cell leukemia and of a special subset of multiple myeloma. Leukemia. 1998; 12: 1977-1982.
- Moreau P, Robillard N, Avet-Loiseau H, Pineau D, Morineau N, Milpied N, et al. Patients with CD45 negative multiple myeloma receiving high-dose therapy have a shorter survival than those with CD45 positive multiple myeloma. Haematologica. 2004; 89: 547-551.
- Moreau P, Robillard N, Jégo G, Pellat C, Le Gouill S, Thoumi S, et al. Lack of CD27 in myeloma delineates different presentation and outcome. Br J Haematol. 2006; 132: 168-170.
- Bataille R, Pellat-Deceunynck C, Robillard N, Avet-Loiseau H, Harousseau JL, Moreau P, et al. CD117 (c-kit) is aberrantly expressed in a subset of MGUS and multiple myeloma with unexpectedly good prognosis. Leuk Res. 2008; 32: 379-382.
- Mateo G, Montalban MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutierrez N, et al. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. J Clin Oncol. 2008; 26: 2737-2744.
- Liebisch P, Eppinger S, Schöpflin C, Stehle G, Munzert G, Döhner H, et al. CD44v6, a target for novel antibody treatment approaches, is frequently expressed in multiple myeloma and associated with deletion of chromosome arm 13q. Haematologica. 2005; 90: 489-493.
- Kumar S, Kimlinger T, Morice W. Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol. 2010; 23: 433-451.
- Greipp PR, Lust JA, O'Fallon WM, Katzmann JA, Witzig TE, Kyle RA. Plasma cell labeling index and beta 2-microglobulin predict survival independent of thymidine kinase and C-reactive protein in multiple myeloma. Blood. 1993; 81: 3382-3387.
- Steensma DP, Gertz MA, Greipp PR, Kyle RA, Lacy MQ, Lust JA, et al. A high bone marrow plasma cell labeling index in stable plateau-phase multiple myeloma is a marker for early disease progression and death. Blood. 2001; 97: 2522-2523.
- Larsen JT, Chee CE, Lust JA, Greipp PR, Rajkumar SV. Reduction in plasma cell proliferation after initial therapy in newly diagnosed multiple myeloma measures treatment response and predicts improved survival. Blood. 2011; 118: 2702-2707.
- Hose D, Rème T, Hielscher T, Moreaux J, Messner T, Seckinger A, et al. Proliferation is a central independent prognostic factor and target for personalized and risk-adapted treatment in multiple myeloma. Haematologica. 2011; 96: 87-95.
- Hose D, Rème T, Meissner T, Moreaux J, Seckinger A, Lewis J, et al. Inhibition of aurora kinases for tailored risk-adapted treatment of multiple myeloma. Blood. 2009; 113: 4331-4340.
- Duhl DM, Renhowe PA. Inhibitors of kinesin motor proteins--research and clinical progress. Curr Opin Drug Discov Devel. 2005; 8: 431-436.
- D'Sa S, Abildgaard N, Tighe J, Shaw P, Hall-Craggs M. Guidelines for the use of imaging in the management of myeloma. Br J Haematol. 2007; 137: 49-63.
- Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD Jr, et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007; 25: 1121-1128.
- Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD Jr, et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007; 25: 1121-1128.
- Hillengass J, Ayyaz S, Kilk K, Weber MA, Hielscher T, Shah R, et al. Changes in magnetic resonance imaging before and after autologous stem cell transplantation correlate with response and survival in multiple myeloma. Haematologica. 2012; 97: 1757-1760.
- Moulopoulos LA, Gika D, Anagnostopoulos A, Delasalle K, Weber D, Alexanian R, et al. Prognostic significance of magnetic resonance imaging of bone marrow in previously untreated patients with multiple myeloma. Ann Oncol. 2005; 16: 1824-1828.
- Bredella MA, Steinbach L, Caputo G, Segall G, Hawkins R. Value of FDG PET in the assessment of patients with multiple myeloma. AJR Am J Roentgenol. 2005; 184: 1199-1204.
- Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011; 118: 5989-5995.
- Bartel TB, Haessler J, Brown TL, Shaughnessy JD Jr, van Rhee F, Anaissie E, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009; 114: 2068-2076.
- Usmani SZ, Mitchell A, Waheed S, Crowley J, Hoering A, Petty N, et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3. Blood. 2013; 121: 1819-1823.
- Zamagni E, Nanni C, Patriarca F, Englaro E, Castellucci P, Geatti O, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007; 92: 50-55.
- Gay F, Larocca A, Wijermans P, Cavallo F, Rossi D, Schaafsma R, et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood. 2011; 117: 3025-3031.
- van de Velde HJ, Liu X, Chen G, Cakana A, Deraedt W, Bayssas M, et al. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007; 92: 1399-1406.
- Hoering A, Crowley J, Shaughnessy JD Jr, Hollmig K, Alsayed Y, Szymonifka J, et al. Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood. 2009; 114: 1299-1305.
- Martinez-Lopez J, Blade J, Mateos MV, Grande C, Alegre A, García-Laraña J, et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood. 2011; 118: 529-534.
- Haessler J, Shaughnessy JD Jr, Zhan F, Crowley J, Epstein J, van Rhee F, et al: Benefit of complete response in multiple myeloma limited to high-risk subgroup identified by gene expression profiling. Clin Cancer Res. 2007; 13: 7073-7079.
- Durie BG, Jacobson J, Barlogie B, Crowley J. Magnitude of response with myeloma frontline therapy does not predict outcome: importance of time to progression in southwest oncology group chemotherapy trials. J Clin Oncol. 2004; 22: 1857-1863.
- Kumar S, Mahmood ST, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Impact of early relapse after auto-SCT for multiple myeloma. Bone Marrow Transplant. 2008; 42: 413-420.
- Jimenez-Zepeda VH, Reece DE, Trudel S, Chen C, Tiedemann R, Kukreti V, et al. Early relapse after single auto-SCT for multiple myeloma is a major predictor of survival in the era of novel agents. Bone Marrow Transplant. 2015; 50: 204-208.
- Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015; 125: 3059-3068.
- Rawstron AC, Gregory WM, de Tute RM, Davies FE, Bell SE, Drayson MT, et al. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. 2015; 125: 1932-1935.
- Kastritis E, Zervas K, Symeonidis A, Terpos E, Delimbassi S, Anagnostopoulos N, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia. 2009; 23: 1152-1157.
- Iriuchishima H, Saitoh T, Handa H, Isoda A, Matsumoto M, Sawamura M, et al. A new staging system to predict prognosis of patients with multiple myeloma in an era of novel therapeutic agents. Eur J haematol. 2015; 94: 145-151.
- Chng WJ, Dispenzieri A, Chim CS, Fonseca R, Goldschmidt H, Lentzsch S, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014; 28: 269-277.
- Ross FM1, Avet-Loiseau H, Ameye G, Gutiérrez NC, Liebisch P, O'Connor S, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica. 2012; 97: 1272-1277.
- Moreau P, Cavo M, Sonneveld P, Rosinol L, Attal M, Pezzi A, et al. Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol. 2014; 32: 2173-2180.