
Research Article
Ann Hematol Oncol. 2015;2(6): 1048.
Immunoglobulin Heavy/Light Chain Quantification – Update on a New Biologic Marker in IgA Multiple Myeloma
Denimal D¹, Lemaire-Ewing S¹, Guy J², Maynadie M³, Bastie JN³, Lafon I³, Caillot D³ and Lakomy D¹*
¹Biochemistry Laboratory, University Hospital Dijon, France
²Haematology Laboratory, University Hospital Dijon, France
³Department of Clinical Haematology, University Hospital Dijon, France
*Corresponding author: Lakomy D, Biochemistry Laboratory, University Hospital Dijon, 2 rue Angelique Ducoudray, 21000 Dijon, France
Received: June 17, 2015; Accepted: July 30, 2015; Published: August 17, 2015
Abstract
In IgA multiple myeloma (MM), monoclonal (M)-spike quantification by serum electrophoresis (SEP) encounters some limitations. Heavy/light chain (HLC) assay is a novel quantitative immunoassay allowing the measurement of IgAk and IgAλ concentrations. The aim of this study was to assess the performances of HLC assay to monitor disease, to detect progression and relapse and to evaluate residual disease in comparison with traditional routine tests.
We compared IgA HLC assay to SEP, immunofixation electrophoresis (IFE), total IgA concentration and bone marrow (BM) immunophenotyping in a routinely follow-up of 40 IgA MM patients (594 serums samples).
HLC assay correlated well with both total IgA and M-spike (when quantifiable) during the follow-up. IgAk/IgAλ ratio monitored progression disease more accurately than SPE. HLC ratio was slightly less sensitive than IFE in the early prediction of relapse. At the stage of complete response (CR), HLC ratio was in perfect agreement with IFE and BM immunophenotyping. Nevertheless, at the stage of near complete response (nCR), IFE and BM immunophenotyping were more sensitive in the assessment of residual disease.
In the current study, IgA HLC assay was found more performant than SPE in the monitoring of partial response and the disease progression but less sensitive than IFE to predict early relapse or to evaluate residual disease.
Keywords: Multiple myeloma; IgA; Heavy/light chain assay; Hevylite
Abbreviations
MM: Multiple Myeloma; SPE: Serum Protein Electrophoresis; M: Monoclonal; IFE: Immunofixation Electrophoresis; IMWG: International Myeloma Working Group; Ig: Immunoglobulin; HLC: Heavy/Light Chain; BM: Bone Marrow; ASCT: Autologous Stem Cell Transplantation; nCR: Near Complete Response; CR: Complete Response; MFC: Multiparameter Flow Cytometry; NR: Normal Range
Introduction
The evaluation of monoclonal (M) protein is a key point of the follow-up in multiple myeloma (MM). Indeed, international guidelines recommend serum protein electrophoresis (SPE) for quantification of monoclonal immunoglobulin (Ig) and immunofixation electrophoresis (IFE) to confirm complete response (CR) [1]. Uniform response criteria for MM are defined by the International Myeloma Working Group (IMWG) based on these well-established laboratory tests [2].
Nevertheless, the interpretation of these conventional laboratory tests can be difficult in IgA MM cases. In particular, an accurate measurement of M-spike by SPE is challenging, due to frequent comigration with other serum proteins in the β-region and/or to the broad electrophoretic migratory patterns [3-5]. IFE is more sensitive than SPE but as a qualitative technique it doesn’t allow quantification. As an alternative, nephelometric quantification of total IgA may be used, but without differentiating monoclonal and polyclonal Ig [2,4].
Since 2009, IgA heavy/light chain (HLC) assay is available providing separate quantification of IgAk and IgAλ and therefore the calculation of the IgAk/IgAλ ratio. This automated immunoassay is based on the recognition of the conformational epitopes at the junctions of the heavy chain and light chain constant regions of Ig [5].
The aim of this study was to evaluate whether the IgA HLC assay meets the criteria of the IMWG and whether it provides similar evaluation of response as the routinely used tests. In that purpose, we compared IgA HLC assay to SPE, IFE, total IgA and bone marrow (BM) analysis. We assessed the potential benefit of IgA HLC assay to monitor disease, to predict disease relapse and progression and to evaluate residual disease.
Materials and Methods
Forty patients presenting an IgA MM under different treatment regimens were routinely followed-up for several months (median 19 months) at the University Hospital of Dijon. Patients were followedup at different times: after induction chemotherapy, after autologous stem cell transplantation (ASCT), after the consolidation phase and during monitoring adapted to the disease evolution.
Sequential samples (594 sera) were kept frozen (<-20°C) until analysis and total IgA quantification, SPE, IFE, IgA HLC were assayed under routine clinical laboratory conditions. Twenty two BM samples were also collected.
IgAk and IgAλ quantification was realized using Hevylite™ reagents on a SPAplus™ analyser (The Binding Site, Birmingham, U.K.). SPE and IFE were performed on a Sebia Hydrasys™ analyser (Sebia, Evry, France). Total IgA were quantified with a dedicated reagent on the Siemens Dade Behring BNII™ nephelometer (Siemens Diagnostics). BM plasma cells were immunophenotyped by a Navios multiparameter flow cytometer (MFC) (Beckman Coulter). Reference values were those proposed by manufacturers.
Response to treatment was evaluated according to the IMWG criteria [2]. In addition, near complete response (nCR) class was used to define response to treatment characterized by negative SPE but positive IFE.
The correlation lines were obtained by linear regression and correlation coefficients were calculated using the Pearson’s test.
Results and Discussion
In MM, response to treatment is monitored by periodic assessment of M-Ig using several laboratory assays, each one with their advantages and their limitations. Our study evaluated IgA HLC assay in comparison with traditional biological markers in order to define which test performs the best at different stages of the disease during the follow-up.
IgA HLC assay and disease monitoring
IgA HLC results were compared with total IgA quantification and electrophoretic M-spike values during the monitoring of 40 IgA MM patients (26 IgAk and 14 IgAλ). The sum IgAk + IgAλ and total IgA concentrations were strongly correlated both in IgAk MM (335 samples, R²=0.962, Figure 1A) and in IgAλ MM (259 samples, R²=0.970, Figure 1B). By using the SPE method, the M-IgA migrated in β-region for 20 patients and in γ-region for the 20 other subjects. In 185 samples, M-spike values and HLC involved isotype concentrations were compared and were found well correlated (R²=0.938, Figure 1C). During the monitoring of all patients, the kinetic of concentrations was found to be similar between HLC involved isotype, M-spike and total IgA. A representative example in one patient with IgAk MM was shown in Figure 1D.
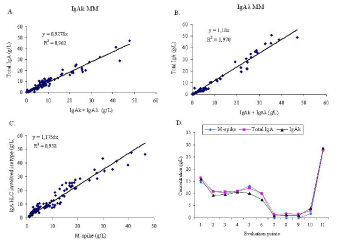
Figure 1: Comparison of total IgA to IgAk + IgAλ in IgAk MM (A) and in IgAλ MM (B). Comparison of electrophoretic M-spike quantification to IgA HLC involved
isotype (C). M-spike, total IgA and HLC IgAk quantification during monitoring of a representative patient with IgAk MM (D).
Abbreviations: MM: Multiple Myeloma; M: Monoclonal; HLC: Heavy/Light Chain
Electrophoretic M-spike quantification is routinely used to define response to treatment. Nevertheless, M-spike quantification depends on the profile of migration of the M-IgA (β- or γ-region), quantity (more or less than 10 g/L) and how narrow is the M band. Several studies reported difficulties in the detection and the accurate measurement of M-IgA by SPE in an important percentage: 46% (26/56 patients) and 50% (34/68 patients) [6,7]. In the study of Boyle et al. SPE bands were quantifiable only in 67% of cases (105/157 samples) [8]. In our study, 50% of patients presented a M-IgA migrating in β-region. In the study of Katzmann et al. considering 30 IgA MM cases with M-IgA migrating in β-region, the HLC assay post-treatment monitoring provided more accurate results than SPE [9]. A good correlation with total IgA was already reported [5,7,9]. In our study, HLC assay correlated well with both total IgA and M-spike (in the cases where the quantification was possible) suggesting that HLC assay provides the same evaluation of the partial response compared to the traditional markers.
IgA HLC assay and evaluation of residual disease
IgAk/IgAλ ratio, IFE and BM immunophenotyping were compared in 11 patients (Figure 2). Among them, 9 patients achieved nCR before ASCT and CR after ASCT. One patient was in nCR after ASCT and evolved in CR at the end of the consolidation phase. One patient achieved nCR after the induction phase and CR before ASCT. In all cases, SPE and total IgA were not contributive: M-IgA was not detectable on SPE, whereas total IgA was below or within the normal values. At nCR stage, IFE was still positive in all the 11 patients showing the persistence of the M-IgA as a slight band. BM immunophenotyping by MFC was also positive, in perfect agreement with IFE while IgAk/IgAλ ratio was already normalized in all the patients. At the next time of evaluation, nCR was followed in all patients by the achievement of CR. Both BM immunophenotyping and IFE became negative, in accordance with the persistent normal IgAk/IgAλ ratio.
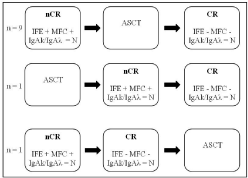
Figure 2: Comparison of IgAk/IgAλ ratio, IFE and bone marrow MFC for the
evaluation of the residual disease.
Abbreviations: nCR: Near Complete Remission; CR: Complete Remission;
IFE: Immunofixation Electrophoresis; MFC: Multiparameter Flow Cytometry;
ASCT: Autologous Stem Cell Transplantation; N: Normal
To evaluate residual disease, IFE known as a more sensitive technique than SPE, allows visualizing residual M-Ig. In addition to serum evaluation, BM immunophenotyping brings valuable information on the residual clone and represents a prognostic tool [10]. By comparing IgAk/IgAλ ratio with IFE and BM immunophenotyping, we found a perfect agreement between these markers at the stage of CR. Nevertheless, at the stage of nCR, IFE was still positive when IgAk/IgAλ ratio was already normalized. Even if IFE requires a technical expertise especially at this stage where M bands are very discrete, the positivity of BM supported the presence of a residual disease. Normal IgAk/IgAλ ratio could be explained in nCR by the increasing proportion of polyclonal Ig associated with a very small amount of residual M-Ig. Few studies suggested a potential role of HLC ratio in the assessment of residual disease [5,6]. Only one congress presentation reported a comparison between HLC ratio and BM immunophenotyping, in accordance with our results [11].
IgA HLC assay and prediction of relapse from CR
During follow-up, 10 patients relapsed (7 patients at different times after ASCT, 1 patient during treatment without graft and 2 patients after several lines of treatment). The kinetic of IFE and HLC ratio was identical in all patients. Table 1 shows, in one representative patient, the results of IFE, SPE, total IgA and IgA HLC assay in sequential testing starting at the moment when IFE became positive again, called T0. When IFE became positive (T0), all the other tests were still negative. The IgAk/IgAλ ratio was the first biological marker that became abnormal after IFE. This abnormal ratio was due to the increase of the involved isotype (IgAλ in the example) while uninvolved isotype (IgAk in the example) was still stable. IgAk and IgAλ concentrations were still in normal range. Later, the involved isotype concentration increased above the normal range while total IgA and SPE were still normal. Then, at the next point of evaluation, total IgA increased out of normal range and M-spike became detectable by SPE, although this one was not quantifiable due to the migration in the β-region. During this time, the IgAk/IgAλ ratio continued to evolve towards more and more abnormal values.
Evaluation points
IFE
SPE
Total IgA
IgAk
IgAλ
IgAk/IgAλ
NR: 0.7- 4.0 g/L
NR: 0.57-2.08 g/L
NR: 0.44-2.04 g/L
NR: 0.78-1.94
T0
Pos again
Neg
1.12
0.57
0.50
1.14
T0+1 month
Pos
Neg
1.20
0.63
0.60
1.05
T0+2 months
Pos
Neg
1.32
0.67
0.72
0.93
T0+4 months
Pos
Neg
2.24
0.93
1.38
0.67
T0+6 months
Pos
Neg
2.68
1.06
2.16
0.49
T0+9 months
Pos
NQ
4.88
0.93
4.16
0.22
T0+10 months
Pos
NQ
5.63
0.82
4.94
0.16
Abbreviations: IFE: Immunofixation Electrophoresis; SPE: Serum Protein Electrophoresis; Pos: Positive; Neg: Negative; NQ: Not Quantifiable; NR: Normal Range. Pathological results are in red.
Table 1: Comparison of IFE, SPE, total IgA and IgA HLC results during relapse (one representative patient out of 10).
Several authors including us reported clinical cases with HLC ratio predicting relapse earlier than IFE [6,12,13]. In the present study we weren’t able to reproduce these observations. In all the 10 studied patients that relapsed, the earliest biological test that turned positive predicting disease relapse was IFE. Nevertheless, the most performant biological marker after IFE to predict a relapse was IgAk/ IgAλ ratio. IgA HLC performances were much better than those of SPE and classical total IgA quantification. IgA HLC ratio was also more useful than the separate quantification of IgAk and IgAλ. The ratio abnormality seems to be the consequence of the involved isotype increase rather than the uninvolved isotype decrease. The fact that IFE was abnormal earlier than HLC ratio could be explained by the persistence of polyclonal Ig at the moment that IFE became positive. Dejoie et al. highlighted difficulties to interpret IFE [14]. Indeed, IFE interpretation is subjective, requires technical experience and could be operator-dependent. In the case of residual disease, early relapse is characterized by thin bands that could be interpreted as negative, positive or even oligoclonal profile. However in our study, IFE interpretation was not ambiguous, the monoclonal bands were thin but well individualized and not confounded with an oligoclonal profile.
IgA HLC assay and prediction of progression
During the study period, 8 patients presented a progressive evolution of the disease. Table 2 shows the results of IFE, SPE, total IgA and IgA HLC assay in sequential testing in one representative patient. The patient was in partial response, with positive IFE during the 11 points of evaluation. SPE was also positive with a very small and even not quantifiable M-spike. In accordance to positive IFE and SPE, IgAk/IgAλ ratio was also positive. At the point 10 of the evaluation, we observed a slight increase in M-spike and total IgA concentration, impossible to interpret as early progression. In the mean time, IgAk/IgAλ ratio showed a clear and non equivocal augmentation from 2.48 to 24.56. At that time, involved isotype (IgAk in the example) concentration increased, whereas uninvolved isotype (IgAλ in the example) concentration started to decrease. Finally, at point 11, the progression was no doubtful with even more pronounced abnormality of HLC assay results. The 7 other patients presented a similar evolution, with a constant abnormal IgAk/IgAλ ratio predicting progression.
Evaluation points
IFE
SPE
Total IgA
IgAk
IgAλ
IgAk/IgAλ
NR: 0.7- 4.0 g/L
NR: 0.57-2.08 g/L
NR: 0.44-2.04 g/L
NR: 0.78-1.94
1
Pos
2.1
2.9
2.52
0.51
4.94
2
Pos
1.7
3.01
2.36
0.51
4.62
3
Pos
1.4
2.09
1.43
0.54
2.65
4
Pos
NQ
2.02
1.31
0.53
2.47
5
Pos
1.3
1.85
1.17
0.51
2.29
6
Pos
1.4
1.87
1.20
0.52
2.30
7
Pos
1.2
1.43
1.21
0.53
2.28
8
Pos
1.0
1.77
1.19
0.52
2.29
9
Pos
1.3
1.91
1.34
0.54
2.48
10
Pos
3.5
6.6
5.65
0.23
24.56
11
Pos
5.3
8.72
7.98
<0,017
>469
Abbreviations: IFE: Immunofixation Electrophoresis; SPE: Serum Protein Electrophoresis; Pos: Positive; NQ: Not Quantifiable; NR: Normal Range. Pathological results are in red.
Table 2: Comparison of IFE, SPE, total IgA and IgA HLC results during progression (one representative patient out of 8).
During disease progression, quantification of electrophoretic M-spike is challenging because the early variations of M-IgA are generally at low concentration and SPE quantification is not always reliable. In contrast, IgA-HLC automated assay is easy to perform and gives a reproductible quantitative value. The role of HLC ratio in the assessment of early progression disease was reported by Bradwell et al. [5]. In our study, IgAk/IgAλ ratio predicted correctly the disease progression in all cases, being more sensitive than SPE. The increase of total IgA is not specific of clonal burden; while the increase of IgA HLC involved isotype in parallel with the decrease of HLC uninvolved isotype suggests an evolution of the tumoral clone.
Conclusion
HLC assay is a reliable alternative to SPE for evaluation of partial response, especially when the precise quantity of the M-IgA is not adequately measured by SPE. It can reliably replace SPE and total IgA measurement without affecting the IMWG definitions of response to treatment. HLC ratio is more sensitive than SPE to accurately monitor progression disease. In our study, HLC assay cannot replace IFE to predict relapse or to evaluate residual disease since HLC ratio, unlike IFE, is not modified by very low concentration of M-Ig in the presence of polyclonal Ig.
Acknowledgement
This work was supported by the Conseil Regional de Bourgogne. We thank Roz Pascale, Pedrocchi Edith, Pacot Agnes and Sommet Dominique for excellent technical assistance.
References
- Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011; 117: 4701-4705.
- Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011; 117: 4691-4695.
- McCudden CR, Mathews SP, Hainsworth SA, Chapman JF, Hammett-Stabler CA, Willis MS, et al. Performance comparison of capillary and agarose gel electrophoresis for the identification and characterization of monoclonal immunoglobulins. Am J Clin Pathol. 2008; 129: 451-458.
- Murray DL, Ryu E, Snyder MR, Katzmann JA. Quantitation of serum monoclonal proteins: relationship between agarose gel electrophoresis and immunonephelometry. Clin Chem. 2009; 55: 1523-1529.
- Bradwell AR, Harding SJ, Fourrier NJ, Wallis GL, Drayson MT, Carr-Smith HD, et al. Assessment of monoclonal gammopathies by nephelometric measurement of individual immunoglobulin kappa/lambda ratios. Clin Chem. 2009; 55: 1646-1655.
- Ludwig H, Milosavljevic D, Zojer N, Faint JM, Bradwell AR, Hübl W, et al. Immunoglobulin heavy/light chain ratios improve paraprotein detection and monitoring, identify residual disease and correlate with survival in multiple myeloma patients. Leukemia. 2013; 27: 213-219.
- Wolff F, Debaugnies F, Rozen L, Willems D, Brohet F, Brauner J, et al. Assessment of the diagnostic performances of IgA heavy and light chain pairs in patients with IgA monoclonal gammopathy. Clin Biochem. 2013; 46: 79-84.
- Boyle EM, Fouquet G, Guidez S, Bonnet S, Demarquette H, Dulery R, et al. IgA Kappa/IgA Lambda Heavy/Light Chain Assessement in the Management of Patients with IgA Myeloma. Cancer. 2014; 1-6.
- Katzmann JA, Willrich MA, Kohlhagen MC, Kyle RA, Murray DL, Snyder MR, et al. Monitoring IgA multiple myeloma: immunoglobulin heavy/light chain assays. Clin Chem. 2015; 61: 360-367.
- Paiva B, Martinez-Lopez J, Vidriales MB, Mateos MV, Montalban MA, Fernandez-Redondo E, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011; 29: 1627-1633.
- Olivero B, Robillard N, Wuilleme S, Avet-Loiseau H, Attal M, Roussel M, et al. Heavy/Light Chain Assay, Potential New Tool in Minimal Residual Disease Assessment, a Biologic Study from IFM 2008 Trial. 13th International Myeloma Workshop. 2011; 3-6.
- Donato LJ, Zeldenrust SR, Murray DL, Katzmann JA. A 71-year-old woman with multiple myeloma status after stem cell transplantation. Clin Chem. 2011; 57: 1645-1648.
- Lakomy D, Lemaire-Ewing S, Denimal D, Bastie JN, Lafon I, Caillot D. [Evaluation of the new HevyliteTM IgA assay for the diagnosis and follow-up of monoclonal gammopathies]. Ann Biol Clin (Paris). 2013; 71: 157-163.
- Caillon H, Dejoie T, Le Loupp AG, Azoulay-Fauconnier C, Masson D, Moreau P, et al. Difficulties in immunofixation analysis: a concordance study on the IFM 2007-02 trial. Blood Cancer J. 2013; 3: e154.