
Case Report
Ann Hematol Oncol. 2015;2(7): 1051.
Two Cases of Donor-Derived Malignancies Following Allogeneic Hematopoietic Stem Cell Transplantation
Fiala MA¹*, Hucthagowder V², Westervelt P¹, Stockerl-Goldstein KE¹, Kulkarni S² and Ghobadi A¹
¹Department of Medicine, Division of Oncology, Washington University School of Medicine, USA
²Department of Pathology and Immunology, Washington University School of Medicine, USA
*Corresponding author: Mark A. Fiala, Department of Medicine, Division of Oncology, Washington University School of Medicine, 660 S. Euclid Avenue, Campus Box 8007, St. Louis MO 63110, USA
Received: July 21, 2015; Accepted: August 10, 2015; Published: August 12, 2015
Abstract
Herein, we present the clinical and laboratory characteristics of two patients who developed donor-derived malignancies following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for acute myeloid leukemia (AML). The first case developed 5q- myelodysplastic syndrome (MDS) approximately 6 months following allo-HSCT. The etiology was inadvertent transfer of cancer cells from the donor to the recipient; we were able to confirm this by performing fluorescent in-situ hybridization (FISH) on a portion of the original pheresis product. The stem cell donor was subsequently diagnosed with 5q- MDS. The second case developed donor-derived AML 23 years post allo-HSCT, the longest reported interval from allo-HSCT to donor-derived malignancy. The etiology of case 2 is unclear but could have occurred due to the presence of oncogenes in the donor cells at transplantation or new somatic mutations that occurred posttransplantation. The stem cell donor has not developed MDS or AML.
Keywords: Donor-derived; Hematopoietic stem cell; Transplantation; Chromosomal abnormalities
Abbreviations
allo-HSCT: Allogeneic Hematopoietic Stem Cell Transplantation; AML: Acute Myeloid Leukemia; MDS: Myelodysplastic Syndrome; FISH: Fluorescence in Situ Hybridization; CLAM: Cladribine, Cytarabine, G-CSF, and Mitoxantrone; MEC: Mitoxantrone, Etoposide and Cytarabine; STR: Short Tandem Repeat; GVHD: Graft-Versus-Host-Disease
Introduction
Reported cases of donor-derived malignancies following allogeneic hematopoietic stem cell transplantation (allo-HSCT) are rare with approximately 60 cases in the literature to date. The estimated incidence is 124 cases per 100,000 allo-HSCT [1]; however, it is likely that the true incidence is much higher as donor-derived malignancies can be mistaken for relapse. Herein, we present the clinical and laboratory characteristics of two patients who developed donor-derived malignancies following allo-HSCT for acute myeloid leukemia (AML).
Case Presentation
Case 1 is a 57-year-old white female was diagnosed with de novo myelodysplastic syndrome (MDS) in 2009. Conventional cytogenetics showed a normal female karyotype (46,XX); fluorescence in situ hybridization (FISH) studies were negative for abnormalities at 5q, 7q, 13q and 20q.The patient was clinically asymptomatic and had not required any transfusions at that point; therefore, treatment was not indicated.
Four months later, the patient had frank progression to AML. Again cytogenetics showed a normal female karyotype; FISH was not performed. She was not initially a candidate for aggressive antileukemic therapy due to poor performance status; therefore, the patient was treated with decitabine but it was discontinued after 4 cycles due to progressive disease. She then received one cycle of CLAM [cladribine, cytarabine, G-CSF, and mitoxantrone] with no response, followed by one cycle of MEC [mitoxantrone, etoposide, and cytarabine]. Twenty days following completion of MEC her marrow did not show any evidence of AML, however, she was pancytopenic, consistent with complete remission with incomplete count recovery.
The patient then proceeded to allo-HSCT on a clinical trial using busulfan and clofarabine conditioning (clinicaltrials.gov NCT01457885). The patient’s HLA-matched 60-year-old brother donated peripheral blood stem cells following mobilization with G-CSF. A total of 10.7x106 CD34+ cells/kg (recipient weight) were collected, and 5.0x106 were infused into the recipient.
Thirty days following allo-HSCT, the recipient’s bone marrow showed no evidence of disease; FISH and short tandem repeat (STR) analysis were consistent with complete donor engraftment, however, FISH showed evidence of deletion of EGR1 at 5q in 21.5% (43/200) of cells analyzed. One-hundred days following allo-HSCT FISH did not show any evidence of 5q-.
The patient suffered from persistent thrombocytopenia following allo-HSCT which was suspected to be secondary to poor graft function and, therefore, she was enrolled on a clinical trial utilizing a CD34+-selected “boost” infusion without any conditioning regimen of immunosuppression (www.clinicaltrials.gov NCT01026987). As part of the trial, her brother, and stem cell donor, underwent an additional mobilization with G-CSF and plerixafor followed by one day of apheresis. A total of 24.6x106 CD34+ cells/kg (recipient weight) were collected, and 13.8x106 were infused into the recipient following CD34+ selection/T-cell depletion. The boost infusion occurred 112 days following allo-HSCT.
Following the boost infusion, the patient had improvement in her platelet counts but she continued to be RBC transfusion dependent. Therefore, a bone marrow biopsy and chimerism studies were performed 77 days following the boost infusion. Bone marrow showed no evidence of disease and STR analysis results were consistent with complete donor engraftment. Conventional cytogenetics showed a male karyotype but revealed a deletion at 5q12q33 in 25% (5/20) of metaphases examined. The diagnosis of 5q- MDS, suspected of donor origin, was made.
Based on the findings, the patient’s brother, and allo-HSCT donor, was contacted to assess for malignancy. At that time he was found to be slightly cytopenic (WBC 2.8k/μL, hemoglobin 12.4g/dL) and, therefore, he underwent a bone marrow biopsy. The bone marrow demonstrated multi lineage dysplasia with no increase in myeloblasts. Conventional cytogenetics showed a complex male karyotype which included 5q deletion; the cytogenetic findings were confirmed by microarray analysis. The diagnosis of 5q- MDS was made.
To confirm inadvertent transfer of 5q- MDS cells from the donor to the recipient, FISH studies were performed on cryopreserved product from both the initial allo-HSCT and from the boost cell infusion. The allo-HSCT and boost CD34-selected products showed evidence of deletion of EGR1 at 5q in 7.5% (15/200) and 15.5% (31/200) of cells, respectively.
The recipient was started on lenalidomide (5mg daily); however, she only received 2 cycles before it was discontinued due to bilateral deep vein thrombosis in her lower extremities. She then suffered from extensive chronic graft-versus-host-disease (GVHD) and renal failure requiring hemodialysis of unclear etiology. Her status was changed to supportive care. The patient expired of sepsis secondary to 5q- MDS 40 months following her allo-HSCT, 34 months following the diagnosis of donor-derived 5q- MDS.
Initially the donor was observed off therapy. Thirty-four months following his diagnosis he became more cytopenic and biopsy showed 8% myeloblasts. Therefore, he was started on lenalidomide 10mg daily and at the time of manuscript preparation had completed 1 year of therapy; he has trilineage hematologic improvement.
Case 2 is a 44-year-old white male who was referred to our institution in 2012 for presumed AML relapse following allo-HSCT in 1989. He had been at his regular state of health until 1 month prior when he started noticing worsening fatigue and decreased energy. Laboratory work-up at that time revealed pancytopenia with a white count of 3.2 k/μL, hemoglobin of 11 g/dl, and platelets of 54 k/μL; blood counts taken 11 months prior were unremarkable.
The patient had originally been diagnosed with AML in 1989, at age 20. At that time, his bone marrow was cytogenetically normal with the exception of trisomy 21 found in 96% (24/25) of cells analyzed; the patient had no clinical manifestation of Down syndrome. He initially underwent 2 cycles of cytarabine induction followed by 1 cycle of high-dose cytarabine and danorubicin for consolidation. He achieved a complete remission with cytogenetics showing a normal male karyotype, but relapsed within 6 months. He then underwent allo-HSCT using his HLA 5/6 match (A antigen mismatch) older brother as a bone marrow stem cell donor.
The patient’s post-transplant course was uncomplicated with the exception of mild acute skin GVHD. The patient developed no obvious chronic GVHD. He had been in remission with full-donor engraftment since the time of his allo-HSCT.
To confirm AML relapse, a bone marrow biopsy and STR analysis were performed to determine disease and chimerism status, respectively. The bone marrow was comprised predominantly of myeloblasts, 57% by flow cytometry, but STR studies indicated fulldonor engraftment. Cytogenetics showed a normal male karyotype with no evidence of the trisomy 21 that was present at initial diagnosis.
The diagnosis of donor-derived AML was made. The patient was scheduled to undergo induction chemotherapy with cytarabine and danorubicin, however, his condition quickly deteriorated due to sepsis and multiorgn failure and the patient expired prior to beginning therapy. The patient’s brother and allo-HSCT donor had not been diagnosed with MDS or leukemia at the time of manuscript preparation, three years following the recipient’s diagnosis.
Discussion
In most reported cases of donor-derived malignancies following allo-HSCT the evolution is indeterminate, but several possible mechanisms have been hypothesized. They include: impaired immune surveillance, transfer of oncogenic material from recipient to donor cells, residual effects of conditioning chemotherapy or radiation, or the inadvertent transfer of cancer cells from the donor to the recipient [2].
In Case 1, the etiology was inadvertent transfer of cancer cells from the donor to the recipient. We were able to confirm this by performing FISH on a portion of the original pheresis products (Figure 1). In this case, the transmission of cancer cells to the recipient likely could have been prevented had the donor undergone a diagnostic bone marrow prior to stem cell donation. One transplant center that required a bone marrow biopsy from their donors over a 1 year period reported finding 3 incidental cases of hematologic malignancies within 99 donors screened [3]. Requiring a bone marrow prior to stem cell donation could have several negative consequences including: increasing costs, delaying transplants, and reducing the population of willing donors.
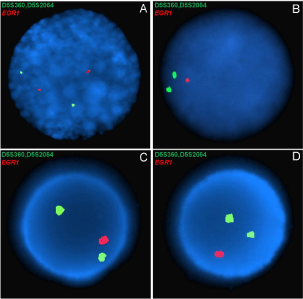
Figure 1: Florescent in situ hybridization results from a dual-color probe
for EGR1 and D5S23/D5S721 from A) the recipient pre-transplant, B) the
recipient post-transplant, C) the donor’s allo-HSCT product, and D) the
donor’s boost infusion product of case 1. The single red fluorescent signal
seen in the recipient post-allo-HSCT (B) and in both donor pheresis products
(C&D) is indicative of deletion of EGR1; the two fluorescent red signals seen
in the recipient prior to allo-HSCT (A) is the normal pattern.
In Case 2, the etiology is less clear and could have occurred by any of the hypothesized mechanisms, although inadvertent transmission of cancer cells would be extremely unlikely as onset occurred 23 years post allo-HSCT. Donor-derived malignancies generally develop within the first few years following allo-HSCT; however, several cases occurring later have been identified. The previous longest reported interval was 14 years [4]. In these cases of late-onset it is unclear if the genetic abnormalities that ultimately lead to the new leukemia diagnosis were transmitted to the recipient from the donor or are new somatic mutations that occurred post-transplantation. Interestingly, at diagnosis the patient had trisomy 21 which has been associated with germline mutations in the hematopoietic transcription factors GATA1 and GATA2, a predisposition for familial leukemia [5]; however, he has no familial history of leukemia or any other cancer.
As donor-derived malignancies following allo-HSCT occur so infrequently the available data originate entirely from analyses of a small number of case reports. However, as awareness increases, the rate of diagnosis will likely also increase. Thus, future analyses could lead to an increase in knowledge about this rare condition.
References
- Hertenstein B, Hambach L, Bacigalupo A, Schmitz N, McCann S, Slavin S, et al. Development of leukemia in donor cells after allogeneic stem cell transplantation--a survey of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2005; 90: 969-975.
- Reichard KK, Zhang QY, Sanchez L, Hozier J, Viswanatha D, Foucar K. Acute myeloid leukemia of donor origin after allogeneic bone marrow transplantation for precursor T-cell acute lymphoblastic leukemia: case report and review of the literature. Am J Hematol. 2006; 81: 178-185.
- Kiss TL, Chang H, Daly A, Messner HA, Jamal N, Spaner D, et al. Bone marrow aspirates as part of routine donor assessment for allogeneic blood and marrow transplantation can reveal presence of occult hematological malignancies in otherwise asymptomatic individuals. Bone Marrow Transplant. 2004; 33: 855-858.
- Dickson MA, Papadopoulos EB, Hedvat CV, Jhanwar SC, Brentjens RJ. Acute myeloid leukemia arising from a donor derived premalignant hematopoietic clone: A possible mechanism for the origin of leukemia in donor cells. Leuk Res Rep. 2014; 3: 38-41.
- Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011; 43: 1012-1017.