
Case Report
Ann Hematol Oncol. 2015;2(7): 1052.
Resolution of Telangiectasia Macularis Eruptiva Perstans with Successful Treatment of Synchronous Large Granular Lymphocytic Leukemia
Saud Alsubait¹, Deeb A3,4, Zimmermann N3,5, Bhaskaran J3, Salkeni MA1,2*
¹Department of Medicine, West Virginia University, USA
²Mary Babb Randolph Cancer Center, West Virginia University, USA
³Cincinnati Veteran Affairs Hospital Medical Center, USA
4Department of Medicine, University of Cincinnati, USA
5Department of Pathology, University of Cincinnati, USA
*Corresponding author: Mohamad A Salkeni, Department of Medicine, Mary Babb Randolph Cancer Center, West Virginia University, Morgantown, USA
Received: July 10, 2015; Accepted: August 09, 2015; Published: August 11, 2015
Abstract
We present a case of an uncommon manifestation of a rare type of cutaneous mastocytosis, Telangiectasia Macularis Eruptiva Perstans (TMEP). The diagnosis of TMEP was made simultaneously with pancytopenia caused by large granular lymphocytic leukemia (LGL). Successful treatment for LGL with a six-month course of oral cyclophosphamide and prednisone led to complete resolution of TMEP skin lesions, in addition to recovery of counts. The World Health organization (WHO) classification of hematopoietic disorders did not include telangiectasia macularis eruptiva perstans as subtype of cutaneous mastocytosis. On the other hand, the classification acknowledged a subtype of systemic mastocytosis that is associated with clonal hematological non-mast cell lineage disease. This case sheds a light on common association of mast cell disorders with other clonal hematological disorders. Pathophysiology is not completely understood and further studies are needed on a larger scale, especially on the molecular level.
Keywords: Cutaneous mastocytosis; Telangiectasia Macularis Eruptiva Perstans; Pancytopenia
Introduction
Cutaneous mastocytosis is defined by the abnormal accumulation of mast cell in the skin. This may or may not lead to significant symptoms. The treatment is generally directed toward symptom control; hence, if asymptomatic, patient may be observed. While there are ample recommendations to guide the treatment of CM, literature guiding therapy for TMEP is scarce. We present a case of a TMEP which completely resolved with the treatment of the associated clonal hematological disorder, which, in our patient, was large granular lymphocytic leukemia.
Case Presentation
A 61-year-old Caucasian male was evaluated simultaneously in dermatology and hematology clinics for asymptomatic skin rash and pancytopenia, respectively. His rash started on his feet approximately seven years earlier and spread gradually to involve his calves, shins, and knees, in addition to chest and abdomen. He reported no associated pruritus or other symptoms of irritation. He has recently noticed increased dyspnea on exertion and fatigue which he attributed to deconditioning. No other constitutional symptoms were reported. A complete blood count performed by patient’s primary care physician revealed pancytopenia. On physical examination, there were numerous scarlet-colored macular and telangiectatic spots that measured 1-3 mm in size. The lesions were confluent, too numerous to count and were mainly located on the shins, knees, lower medial thighs, lower abdomen (peri-umbilical), and the chest (periareolar) (Figures 1A,1B,1C). Patient was found to have a palpable splenic tip and palpable liver 2 cm below costal margin. Laboratory work up on initial presentation revealed mild anemia with inadequate reticulocytosis, thrombocytopenia (hematocrit 29.3%, hemoglobin 10.3 g/dl, MCV 98.3 fL, platelets 102,000 per μL), and significant leukopenia and neutropenia (WBC 1,300 per μL, absolute neutrophil count 488 per μL, and absolute lymphocyte count 447 per μL). On further testing, patient was found to have elevated conjugated and total bilirubin (0.5 and 2.1 mg/dL. Normal range ≤ 0.3 and 0.2- 1.2 for direct and total bilirubin, respectively), elevated alkaline phosphatase (249 U/L. Normal range35-110 U/L) and slight elevation in ALT and AST (51 U/L and 47 U/L. Normal range 0-50 U/L and 5-40 U/L for ALT and AST, respectively). Review of peripheral blood smear was most significant for the presence of occasional large lymphocytes with abundant cytoplasm that contained few large granules (Figure 2). Anemia work up ruled out the presence of any nutritional deficiency.
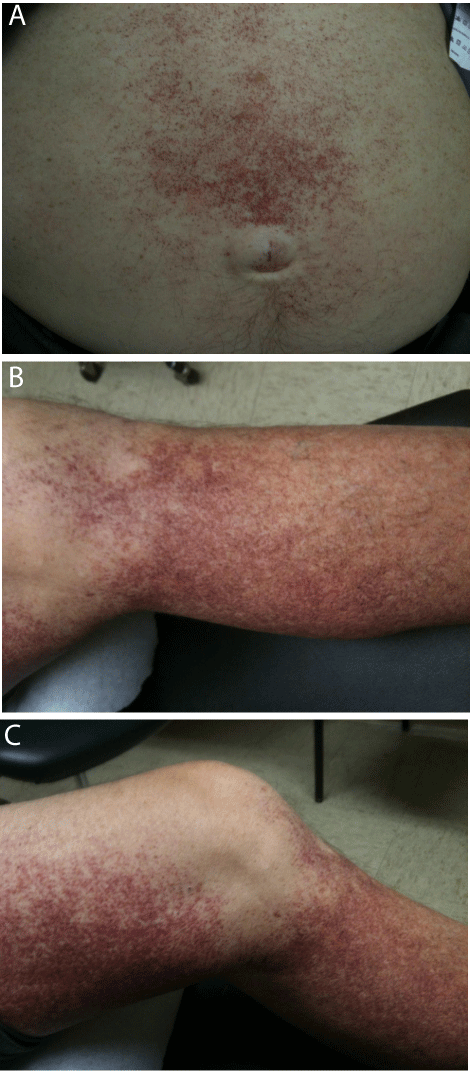
Figure 1: Skin photographs showing rash prior to any treatment.
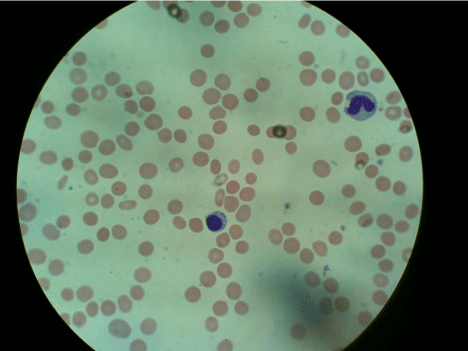
Figure 2: Peripheral blood smear.
Skin biopsy of the periumbilical area revealed telangiectatic lesion with perivascular CD117 positive mast cell infiltration, suggestive of Telangiectasia Macularis Eruptiva Perstans (TMEP) (Figures 3A,3B,3C,3D).
A bone marrow aspiration and biopsy was subsequently performed and revealed normal cellularity (~40%), relative erythroid hyperplasia, decreased granulopoiesis, adequate megakaryopoiesis and no evidence of systemic mastocytosis or myelodysplasia. No collections of atypical mast cells were identified on H&E staining, by mast cell tryptase and CD117 immunostains, or by flow cytometry. Bone marrow kayrotyping revealed a normal male kayrotype (46, XY).
Flow cytometry of peripheral blood, as well as of bone marrow aspirate, revealed the presence of atypical lymphocytes that were uniformly CD8 positive, with absent CD56 and CD57 and partial expression of CD7 and CD16. This population of cells made up about 10% of the leukocytes. They were thought to represent an atypical population of T-large granular cells. Their significance was not certain but raises the possibility of a chronic lymphoproliferative disorder of T/NK cells given the uniform expression of CD8 [1].
A head to thigh positron emission tomography combined with contrast-enhanced computed tomography (PET/CT) did not show any abnormal lymphadenopathy; however, the spleen was noted to be mildly to moderately enlarged.
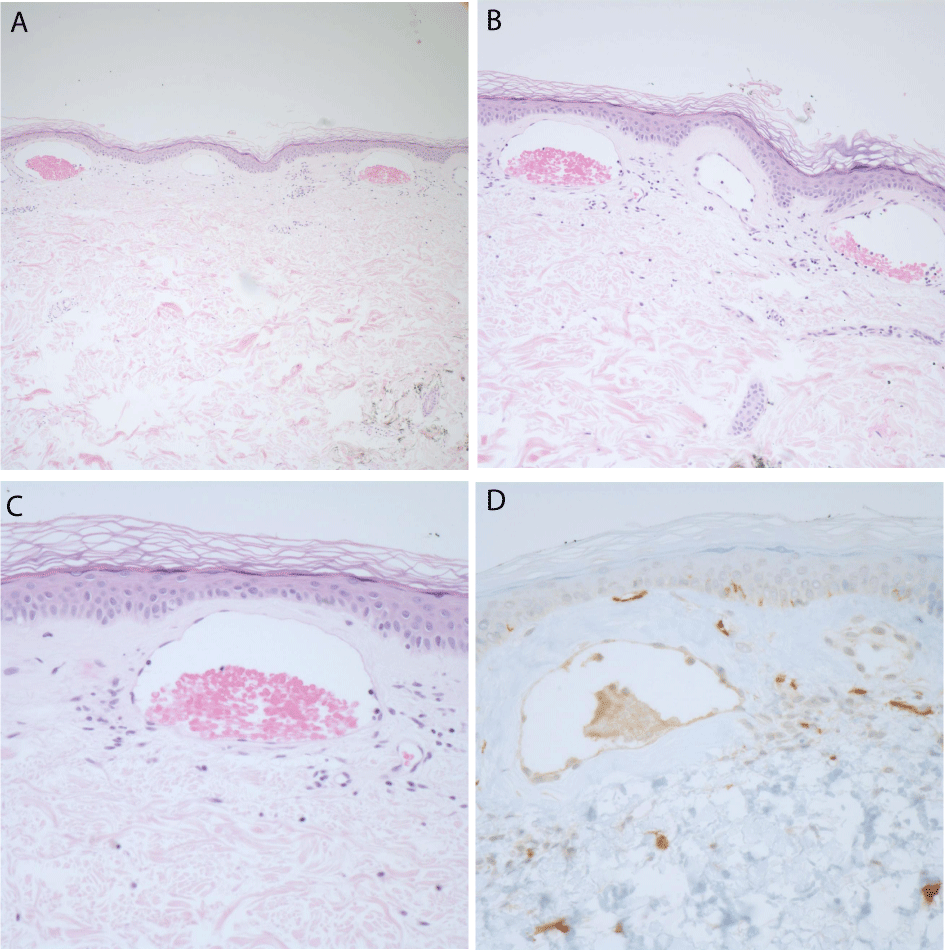
Figure 3: Skin punch biopsy with dilated vascular spaces immediately below
the epidermis.
Liver biopsy was performed and revealed, by immunostains and flow cytometry, increased sinusoidal lymphocytes with phenotype similar to the above described population (Figures 4A,4B). These cells were positive for CD8, CD16, and TIA-1 and negative for CD56 and CD57. There were no significant hepato cellular damage. Immunostains for EBV, CMV, and HSV were negative.
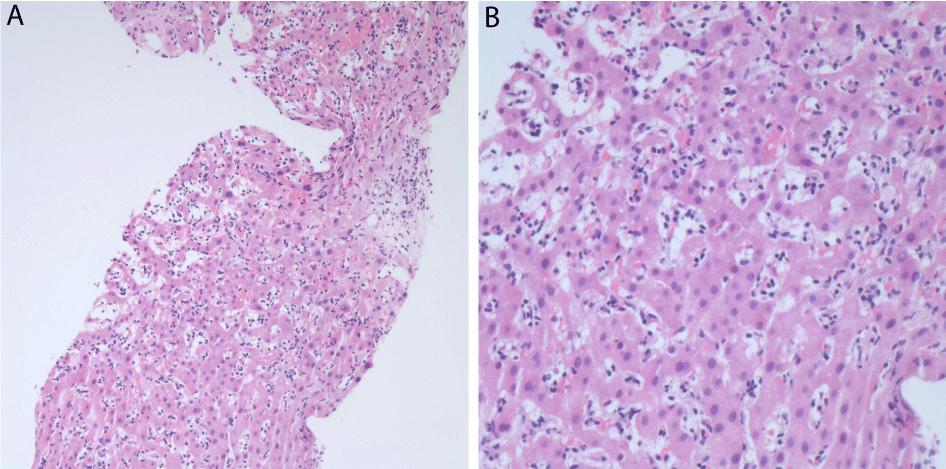
Figure 4: Congested sinusoids, filled with small bluish-looking lymphocytes.
Figure 4A: Liver biopsy 20Xmagnification.
Figure 4B: Liver biopsy 40Xmagnification.
Further laboratory work up revealed normal serum tryptase levels and no evidence of viral hepatitis. Detection of C-KIT D816V mutation in the bone marrow was negative by polymerase chain reaction (PCR). Lactate dehydrogenase (LDH) was elevated at 418 U/L (normal range 90-220 U/L). Prothrombin and partial thromboplastin time’s and haptoglobin levels were within normal limits.
Upon presentation, patient was minimally symptomatic; hence, he was followed for a period of about 6 months. However his hemoglobin gradually dropped to levels below 7 gm/dL and he became increasingly fatigued and dyspneic on minimal exertion to the point of interfering with activities of daily living. Hence, after discussion with patient, decision was to initiate therapy of LGL. After review of multiple treatment options for LGL, we elected to proceed with daily oral cyclophosphamide 100 mg daily in addition to a tapering dose of oral prednisone [2,3].
Patient received a total of six 28-day cycles. He tolerated treatment very well with no complications. His hemoglobin and hematocrit recovered to near normal levels (12.6 gm/dL and 38.4%, respectively). Absolute neutrophil count and platelet recovered to 1760 and 163 per μL, respectively. All skin lesions faded significantly during treatment (Figure 5), and resolved completely one month after treatment was complete.
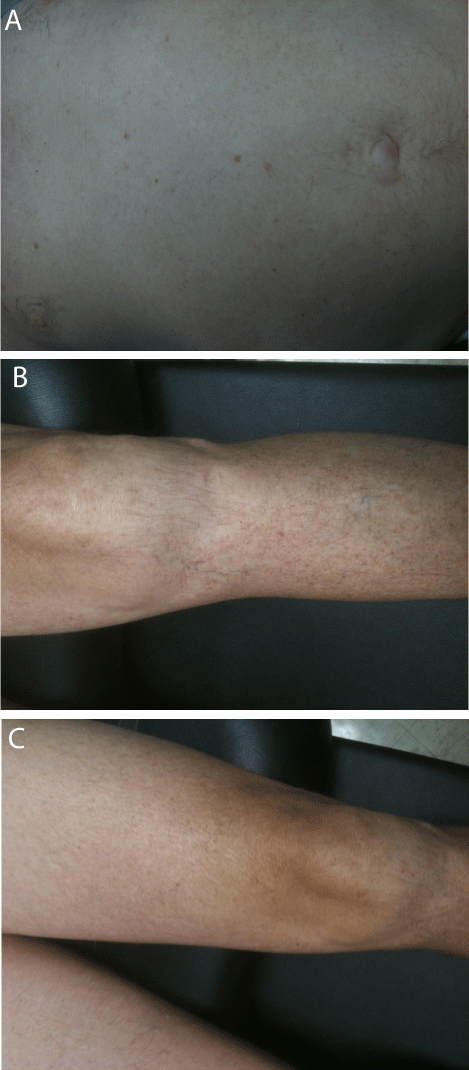
Figure 5: Post treatment skin photographs.
Figure 5A: Periumbilical area.
Figure 5B: Left lower extremity.
Figure 5C: Left lower extremity.
Patient continued to do well post treatment completion, he was commenced on active surveillance with every three months follow up in the hematology clinic. He has remained in remission with no signs of clinical or laboratories relapse for more than three years from initiation of chemotherapy.
Discussion
The presumed pathophysiology of telangiectasia maculariseruptivaperstans (TMEP) is excessive accumulation of mast cells in the dermis. This may lead to the development of skin lesions that may cause significant symptoms, such as pruritus, or be asymptomatic [4].
To the best of our knowledge, there is currently no gold standard treatment for TMEP. Several treatment options have been suggested for symptomatic patients. Symptoms of particular concern are pruritus, which is addressed by antihistamines or mast cell stabilizers. Avoidance of provocative events, such as certain foods, drugs or allergens, is an important first step. Oral H1 and H2 blockers as well as oral cromolyn sodium may help with skin irritative symptoms such as pruritis and urticarial eruptions. Topical glucocorticosteroids can be used for limited symptomatic lesions [5-7]. For more extensive skin lesions UVB phototherapy has been used and shown to have longerlasting benefits compared with oral antihistamines. The treatment of TMEP with with photo-chemotherapy with psoralen UVA (PUVA) in a case report, showed promising results as well. Mechanism of action is presumed to be decreasing mast cell histamine release and decreasing number of cells in the dermis [8,9]. The use of total skin electron beam (TSEB) radiation has also been described with good outcome [10].
Laser surgery using 585-nm flash-pumped dye laser has been reported to be beneficial in one patient for cosmetic reasons with very good short-term results and no scarring. However, most lesions recurred within 14 months of treatment [11]. The leukotriene antagonists, such as montelukast, have been used for a pediatric case and showed promising results. The patient started taking montelukast at the age of 2 and was free from any new lesions for about 2.5 years [7].
It is worth mentioning here that there are several reported cases which resolved spontaneously and others which did not require any treatment.
On a different level and due to risk of anaphylaxis as a result of sudden massive histamine release, patients need to be educated and provided with epinephrine injection kit for use in cases of emergency. These patients may be at risk for anaphylaxis and death due to bronchospasm, swelling, hypotension, and shock [12].
The most recent WHO classification of hematopoietic disorders did not include telangiectasia macularis eruptive perstans as subtype of cutaneous mastocytosis [13]. On the other hand, the classification acknowledged a subtype of systemic mastocytosis that is associated with clonal hematological non-mast cell lineage disease such as myelodysplasia, myeloproliferative disorders, acute leukemia, lymphoma, or other hematological neoplasm (Systemic Mastocytosis with Systemic mastocytosis with associated clonal hematological non-mast cell lineage disease). Vannorsdall et al., reported a case of cutaneous mastocytosis associated with chronic myelogenous leukemia (CML). Treatment of CML by imatinib led to improvement in cutaneous mastocytosis [14].
Conclusion
To our knowledge, this is the first report of TMEP that is associated with a clonal hematological disorder. Even more intriguing is the resolution of most skin lesions along with recovery of counts. This strongly suggests a linkage between TMEP incidence and underlying LGL.
It is noteworthy that most of the studies lack long term follows up. Our patient continued to do well with no clinical evidence of recurrence for over 2.5 years.
Needless to say that large scale studies are needed to make a strong recommendations regarding definitive treatment of TMEP. This may be quite difficult given the rarity of this disease.
Based on the case presented above, we recommend observation alone for asymptomatic patients. We recommend symptom-directed therapy for patients with mild irritative symptoms. More severe symptoms may be considered for UVB, PUVA, or TSEB. Special attention should be paid to any associated hematological abnormalities and if present, rigorous work up need to be conducted to rule out any associated clonal hematological disease. The treatment of associated hematologic disorder, if warranted, may lead to resolution of TMEP.
Although not completely understood, it is apparent that treatment of underlying systemic hematologic disorder led to resolution of cutaneous mastocytosis. How the two entities interact is quite puzzling. Several questions remain unanswered, such as whether treatment had directly led to TMEP regression, or it was indirectly effective via treating underlying hematologic condition.
References
- Dhodapkar MV, Li CY, Lust JA, Tefferi A, Phyliky RL. Clinical spectrum of clonal proliferations of T-large granular lymphocytes: a T-cell clonopathy of undetermined significance? Blood. 1994; 84: 1620-1627.
- Lacy MQ, Kurtin PJ, Tefferi A. Pure red cell aplasia: association with large granular lymphocyte leukemia and the prognostic value of cytogenetic abnormalities. Blood. 1996; 87: 3000-3006.
- Fujishima N, Sawada K, Hirokawa M, Oshimi K, Sugimoto K, Matsuda A, et al. Long-term responses and outcomes following immunosuppressive therapy in large granular lymphocyte leukemia-associated pure red cell aplasia: a Nationwide Cohort Study in Japan for the PRCA Collaborative Study Group. Haematologica. 2008; 93: 1555-1559.
- Ragi J, Lazzara DR, Harvell JD, Milgraum SS. Telangiectasia macularis eruptiva persians presenting as island sparing. J Clin Aesthet Dermatol. 2013; 6: 41-42.
- Costa DL, Moura HH, Rodrigues R, Pineiro-Maceira J, Ramos-E-Silva M. Telangiectasia macularis eruptiva perstans: a rare form of adult mastocytosis. J Clin Aesthet Dermatol. 2011; 4: 52-54.
- Monahan TP, Petropolis AA. Treatment of telangiectasia macularis eruptiva perstans with total skin electron beam radiation. Cutis. 2003; 71: 357-359.
- Cengizlier R, Hücümenoğlu S, Ozen A, Tülin Sayli R. Treatment of telangiectasia macularis eruptiva perstans with montelukast. Allergol Immunopathol (Madr). 2009; 37: 334-336.
- Gollhausen R, Kaidbey K, Schechter N. UV suppression of mast cell-mediated wealing in human skin. Photodermatol. 1985; 2: 58-67.
- Sotiriou E, Apalla Z, Ioannides D. Telangiectasia macularis eruptive perstans successfully treated with PUVA therapy. Photodermatol Photoimmunol Photomed. 2010; 26: 46-47.
- Nisce LZ, Safai B, Kim JH. Electron-beam therapy for mycosis fungoides. J Dermatol Surg Oncol. 1978; 4: 594-599.
- Ellis DL. Treatment of telangiectasia macularis eruptiva perstans with the 585-nm flashlamp-pumped dye laser. Dermatol Surg. 1996; 22: 33-37.
- Górska A, Niedoszytko M, Lange M, Chełmińska M, Nedoszytko B, Wasąg B, et al. Risk factors for anaphylaxis in patients with mastocytosis. Pol Arch Med Wewn. 2015; 125: 46-53.
- Swerdlow SH, Cancer IAfRo, Organization WH. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues: International Agency for Research on Cancer; 2008.
- Vannorsdall EJ, Collins JA, Chen QC, Sarai G, Baer MR. Symptomatic response to imatinib mesylate in cutaneous mastocytosis associated with chronic myelomonocytic leukemia. Curr Oncol. 2013; 20: 349-353.