
Review Article
Ann Hematol Oncol. 2015; 2(7): 1055.
Gemtuzumab Ozogamicin Combined with Induction Chemotherapy in Young Adults with Acute Myeloid Leukemia: Review and Perspectives
Candoni A¹*, Fanin R¹ and Baccarani M²
¹Division of Hematology and SCT Unit, University of Udine, Italy
²Department of Hematology & Oncology, University of Bologna, Italy
*Corresponding author: Anna Candoni, Division of Hematology and Stem Cell Transplantation Unit, Santa Maria Misericordia University Hospital, University of Udine, 33100 Udine, Italy
Received: September 10, 2015; Accepted: November 20, 2015; Published: November 22, 2015
Abstract
Progress in treatment of acute myeloid leukemia (AML) is slow. Many new agents have been tested, but few were approved. Gemtuzumab Ozogamicin (GO) is a new AML-targeted drug that is composed by a monoclonal antibody targeting a surface antigen of myeloid leukemic cells (CD33) combined with a potent cytotoxic (calicheamicin). We review here the studies of GO in AML, including an update of the Italian studies, and we trace back the story of a drug that was developed 15 years ago and, regrettably, is no longer available for the treatment of AML, with the exception of Japan. GO was approved by the US FDA for the second-line treatment of AML in the elderly, and was shown by several European large prospective and randomized studies to be active also in first line, both alone, but particularly in combination with standard chemotherapy. Regrettably, a registration study that was performed in US could not confirm the superiority of GO and chemotherapy on chemotherapy alone, and the drug was withdrawn. The differences among the US and the European studies are discussed. The profile of the AML patients who are expected to benefit more by the reintroduction of GO is proposed: first-line, less than 60 years old, CD33 expressed in more than 20% leukemic cells, low/intermediate cytogenetic risk, and low expression of the PGP multidrug resistance protein.
Keywords: Acute myeloid leukemia; Gemtuzumab ozogamicin; Induction therapy; Multidrug resistance
Introduction
The therapy of many haematologic malignancies has improved dramatically in the last 30 years, but the therapy and the outcome of therapy of acute myeloid leukemia (AML) have changed only partially. Less than five new drugs have been approved for AML in the past 25 years (Gemtuzumab-ozogamicin, decitabine, azacitidine) [1,2].
Recent efforts to improve the efficacy of the therapy for remission induction have included several potential strategies such as modulation of anthracyclines and cytarabine doses, addition of multidrug resistance1 (MDR) modulators, and the use of targeted agents, sd Gemtuzumab-ozogamicin (GO) [1,3,4]. Particularly, GO has been investigated primarily as mono therapy (9 mg/sqm as a standard dose every 2 weeks) in elderly patients with relapsed disease, with a reported overall response rate (ORR) of 25-35%, and a median duration of response shorter than 8 months [5-9]. Based on these results, GO obtained approval, in May 2000, by the United States (US) Federal Drug Administration (FDA) for treatment of patients 60 years old, or older, with AML in first relapse who are not candidates for intensive cytotoxic therapy [9-11]. Then, the effectiveness of GO was also evaluated as first induction monotherapy, always in the elderly. More recently, some randomized trials with addition of GO to induction chemotherapy were performed confirming the potential role and the interest of GO in an induction setting [4-7,12-16].
Here, we review the studies testing GO for the induction of first remission in the specific setting of young adult patients with AML. In this context we also summarize the final results of a multicenter Italian trial where GO was combined with a FLAI scheme (fludarabine, cytarabine, and idarubicin) as induction strategy in AML patients less than 65 years old.
Gemtuzumab-Ozogamicin-Structure and Mechanism of Action
Gemtuzumab-ozogamicin (CMA-676; Mylotarg®) is a chemotherapy agent composed of a recombinant humanized anti- CD33 antibody (IgG4) conjugated with a cytotoxic antitumor antibiotic, calicheamicin, isolated from fermentation of a bacterium, Micromonospora echinospora ssp. Calichensis (Figure 1). The antibody portion of GO binds specifically to the CD33 antigen, a 67-kDA sialic acid-dependent adhesion protein expressed on the surface of leukemic blasts in more than 90% of AML but not on normal hematopoietic stem cells. The expression of CD33 peaks in promyelocytes and myelocytes, and is down regulated with maturation of myeloid lineage (mature granulocytes do not express CD33). The binding of the anti-CD33 antibody portion of GO with the CD33 antigen results in the formation of a complex that is rapidly internalized and transferred into lysosomes. Calicheamicin is then released with subsequent hydrolysis and binds in a sequence-specific manner the minor groove of DNA, resulting in DNA double strand breaks, cell cycle arrest and apoptosis.
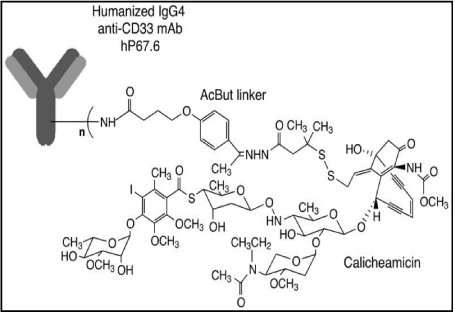
Figure 1: Schematic structure of GO.
Of interest, at low concentrations (0.01-0.025 ng/mL), the in vitro sensitivity of AML cells to GO correlates with CD33 expression, but at high concentrations (1-10 μg/mL), the GO uptake may be CD33- independent.
After administration of the dose that was initially identified as maximum tolerated does, and then approved, that is to say 9 mg/ sqm given as a 2 hour infusion, the elimination half lives of total and unconjugated calicheamicin were about 41 and 143 hours, respectively. After a second 9 mg/sqm dose, the half life of total calicheamicin was increased to about 64 hours and the area under the concentrationtime curve (AUC) was about twice that in the first dose period. This is probably due to decreased clearance by CD33 positive blast cells in case of successful first-dose administration. GO is eliminated mostly by the hepatobiliary system and age, gender, body surface area, and weight were not reported to affect pharmacokinetics [17,18].
Main Clinical Trials including Addition of GO to Induction Chemotherapy in Young Adults with AML
The five studies described below and summarized in Table 1 provide important information regarding efficacy and toxicity of GO combined with intensive chemotherapy in the first line setting in younger AML patients. All but one of these studies showed that the addition of GO improved survival in a subset of patients with newly diagnosed AML when added to conventional induction chemotherapy (Table 1). Specifically, there were four European prospective studies in which 2744 patients were randomized.
REFERENCE
STUDY
Sigle
STUDY
type
Eligibility criteria
Median age
(age group or range)
N° Pts
Induction Scheme
Cumulative
GO dose (mg/m2)
Liver toxicity
III°- IV°
CR rate
DDI
Overall Benefit
on AML Outcome
Burnett AK,
JCO 2011
MRC/NCRI AML15
R (GO vs No GO)
AML de novo or secondary, aged < 60
49
(< 60)
556
vs
557
DA vs ADE vs FLAG-IDA±GO
day 1
3
NA
82%
vs
83%
7%
vs
6%
YES
Burnett AK,
JCO 2012
MCR/NCRI AML16
R (GO vs No GO)
AML de novo or secondary or HR MDS
67
(51-84)
559
vs
556
DA±GO day 4
vs DClo±GO day 4
6
4%
vs
4%
62%
vs
58%
12%
vs
11%
YES
Castaigne S,
Lancet 2012
ALFA 0701
R (GO vs No GO)
AML de novo, aged 50-70
62
(50-70)
139
vs
139
DA±GO
days 1,4,7
9
13%
vs
8%
81%
vs
75%
6,5%
vs
4%
YES
Petersdorf SH, Blood 2013
SWOG S0106
R (GO vs No GO)
AML de novo, aged 18-60
47
(18-60)
277
vs
229
DA**±GO
day 4
6
NA
66%
vs
69%
5,8%
vs
0,8 %*
NO
Delaunay,
Blood 2011 (abs)
GOELAMS AML2006 IR
R (GO vs No GO)
AML de novo, aged 18-60
50
(18-60)
119
vs
119
DA±GO
day 4
6
22%
vs
11,5%
92%
vs
87%
4,2%
vs
2,5%
YES
Candoni, Haematologica 2014 (abs)
GO-FLAI Trial
NR
AML de novo or secondary, aged < 65
52
(18-65)
130
FLAI+GO
day 6
3
1%
82%
3 %
YES
*P=0,002; **(Daunorubicin 45 mg/sqm in GO Arm vs 60 mg/sqm in control Arm)
R: Randomized trials; NR: Not Randomized trial. NA: Not Available.
DA: Daunorubicin, Cytarabine; IDA: Idarubicin, FLAI: Fludarabine, Cytarabine, Idarubicin; Dclo: Daunorubicin, Clofarabine;
DAE: DA plus Etoposide.
Table 1: Main clinical studies including gemtuzumab ozogamicin in induction chemotherapy in young adult aml patients.
In the MRC/NCRI AML15 trial, 1,113 adult patients with de novo AML, were randomized to receive or not a single low dose of GO (3 mg/ sqm) on day 1 of induction course with one of three different induction regimens (cytarabine/daunorubicin/etoposide, daunorubicin/ cytarabine, fludarabine/ cytarabine/G-CSF/idarubicin). As reported in Table 1, there were no differences in the complete remission (CR) rate and in death during induction (DDI) between those patients who did and those who did not receive GO. However, a subgroup analyses showed that the addition of GO improved 5-year overall survival (OS) in patients with favorable cytogenetics (79% vs. 51%; p=0.0003) but not in those with unfavorable cytogenetics (8% vs. 11%; p=0.4). In addition, this study showed that GO could improve survival for some intermediate-risk patients [19].
In the GOELAMS AML 2006 IR study, 238 adults patients, aged 18–60 years, with de novo AML and intermediate karyotype, received daunorubicin/cytarabine with or without GO (6 mg/sqm) on day 4; GO was also added to consolidation therapy according to the initial randomization. There was no difference in overall response rate (ORR) or DDI between the two treatment arms, and GO did not increase toxicity. Overall, there was also no statistically significant difference in event free survival (EFS) (GO vs. control: 51% vs. 33%) or OS (53% vs. 46%) at 3 years. However, subgroup analyses showed that in patients who did not undergo allogeneic hematopoietic stem cell transplantation (allo SCT), EFS was significantly higher in the GO group (54% vs. 27%, p=0.03) [20].
In the ALFA 0701 trial, 278 patients, aged 50–70 years, received daunorubicin/cytarabine with or without GO (3 mg/sqm) on days 1, 4, and 7. A second course of daunorubicin/cytarabine was given on day 15, in case of residual disease. Patients in remission then received 2 courses of daunorubicin/cytarabine with or without GO (3 mg/sqm) on day 1 of each cycle. While there was no statistically significant difference in ORR and DDI between the 2 arms, the eventfree survival (EFS) at 2 years was significantly superior in the GO arm (41% vs. 17%, p=0.0003), as disease-free survival (DFS) (50% vs. 23%, p=0.0003) and OS (53% vs. 42%, p=0.037). Subgroup analysis showed that the EFS benefit occurred in patients with favourable/intermediate cytogenetics but not in those with an adverse karyotype [21].
Finally, in the MRC/NCRI AML16 trial, 1,115 adults, aged 51–84 years, with AML or high-risk myelodysplastic syndrome (defined as >10% marrow blasts at diagnosis) received either daunorubicin/ cytarabine or daunorubicin/clofarabine for 2 cycles with or without GO (3 mg/sqm) on day 1 of the first induction course. Similar to the previous reported trials, there was no significant difference in ORR and DDI between the two treatment arms. However, the administration of GO was associated with reduced relapse risk (at 3 years: 68% vs. 76%; p=0.007) and superior DFS (21% vs. 16%; p=0.04) as well as OS (25% vs. 20%; p=0.05).
A later meta-analysis of AML15 and AML16 trials on 2,224 patients showed a significant benefit of GO for risk of relapse (odds ratio [OR]: 0.82 [95% confidence interval: 0.72–0.93], p=0.002) and survival (OR: 0.88 [0.79–0.98], p=0.02). This survival benefit was clear in patients with favourable (OR: 0.47 [0.28–0.77]) and intermediaterisk (OR: 0.84 [0.73–0.97]) but not with poor-risk (OR: 1.02 [0.81– 1.27]) disease [4,22].
Of note, the safety and efficacy of GO combined with induction chemotherapy was also clearly demonstrated, by the Children’s Oncology Group in pediatric AML and, in both AAML0391 (350 pts) and AAML05331 (1070 pts) trials, GO was associated with increased EFS and RFS rates [23,24].
Unfortunately, the Southwest Oncology Group (SWOG) study-S0106, which was designed and performed, in accordance with the company sponsor (Pfizer), to expand the GO indications after the first FDA approval, failed to confirm and reproduce the European studies (Table 1). In that trial, 637 patients, aged 18–60 years, with de novo AML, were randomized to receive an induction chemotherapy with daunorubicin/cytarabine with or without a single dose of GO (6 mg/sqm) on day 4 of the induction [25]. Unlike the European studies, in which identical doses of conventional chemotherapy were used in both arms, S0106 used a lower daunorubicin dose with GO (45 mg/ sqm vs. 60 mg/sqm), a difference that is likely to be critical. Overall, study S0106 showed no benefit in ORR or OS with the addition of GO, but a trend towards improved OS was seen in patients with favourable risk leukemias (hazard ratio: 0.49 [0.12–2.04]: Regrettably, S0106 was not powered to detect outcome differences in this patient subset. Nonetheless, based on unfavourable outcome of S0106 trial, specifically the lack of OS benefit in the entire study cohort and the increased rate of early (30-day) mortality in the GO arm (6% vs. 1%), this study was prematurely terminated and, following discussions with the FDA, Pfizer voluntarily withdrawned GO from the United States and European markets (June 2010) before the final results of other phase III trials (ALFA 0701, MRC-AML15) were available. Conversely, the drug continues to be commercially available in Japan, where it has received full regulatory approval [18,26,27].
The Italian Multicenter Study of GO and FLAI for Remission Induction in AML Patients less than 65 Years
In four Italian Haematologic Centres, between 2007 to 2010, a prospective multicenter, phase 2, single-arm, clinical trial (NCT 00909168) with GO incorporated in induction chemotherapy (FLAI scheme) in patients younger than 65 years with newly diagnosed AML (FLAI-GO Induction scheme), was performed. This study has now a follow up of more than 5 years [12,13].
The main objective of this prospective multicenter study was to evaluate feasibility, efficacy and toxicity of an induction scheme including low dose of GO (3 mg/sqm) combined with a Fludarabine based regimen (FLAI) in patients 18-65 years old with previously untreated and CD 33 positive AML.
One hundred thirty consecutive patients were included with a median age of 52 years (range 18-65). CD33 expression exceeded 20% in all cases, 25% of patients (31/123 evaluable cases) had an adverse karyotype, 25% (33/130) were secondary AML, and 22% (27/120 evaluable cases) had a Multidrug-Resistance (MDR) phenotype with a P-glycoprotein (PGP) over-expression on blast cells. FLAI-GO regimen included fludarabine 25 mg/sqm (days 1–5), cytarabine 2 g/ sqm (days 1–5), idarubicin 10 mg/sqm (days 1, 3 and 5), and single dose of GO 3 mg/sqm on day 6. Patients were evaluated for response rate and treatment-related adverse events. After induction with FLAIGO, CR rate was 83% (104 of 126 evaluable pts); five patients achieved partial remission (PR) with residual blast cells on bone marrow between 5 and 10% and 17/130 patients were primary resistant, with an ORR of 87%. There were only 4 cases of DDI (3%)-Table 1.
The hematological and extra-hematological toxicity of FLAI-GO was comparable to FLAI alone as reported in our previous studies (Table 2 A/B) [13]. Of note, 45% of patients experienced transient and reversible GO infusion-related adverse events (especially fever and chills), but no cases of veno-occlusive disease (VOD) occurred during chemotherapy or after allo SCT. After induction patients received consolidation therapy with cytarabine (2g/sqm days 1-5) and idarubicin (12 mg/sqm, days 1-3) followed, after the hematological recovery, by high dose of cytarabine (6g/sqm, days 1-4) and allo SCT when indicated (high risk AML). The probability of 2 and 5-year OS was 63% and 52%, respectively. The probability of 2 and 5-year DFS was 54% and 47% respectively. Allogeneic SCT was performed in 58 patients (44%) and auto SCT in 24 (18%) cases.
PMN > 0.5 x109/L
Mean ± SD, days
Median (range), days
23 ± 3.3
23(19-32)
PRC, No.
Mean ± SD,
Median (range)
12 ± 4.5
12(6-26)
PMN > 1 x109/L
Mean ± SD, days
Median (range), days
25 ± 4.7
23(19-39)
PU, No.
Mean ± SD
Median (range)
7.3 ± 3.6
7(3-15)
PLT > 20 x109/L
Mean ± SD, days
Median (range), days
23 ± 4
22(18-38)
G-CSF vials
Mean ± SD
Median (range)
6 ± 6.2
8(0-18)
PLT > 50 x109/L
Mean ± SD, days
Median (range), days
26 ± 5
24(18-43)
Hospitalization
Mean ± SD, days
Median (range), days
30 ± 7
31(22-59)
Table 2A: Italian experience with go (3mg/Sqm) +FLAI. A) Hematologic and B) Extrahemoltologic toxicities (Candoni 2014).
FUO
68/130 (52%)
HSV infectious
24/130 (18%)
BACTEREMIA
34/130 (26%)
PNEUMONIA
22/130 (17%)
(5 mycosis)
MUCOSITIS (II-IV°
WHO)
Grade II°
Grade III°
Grade IV°
22/130 (17%)
20/130 (15,5%)
2/130 (2,5%)
0/130 (0%)
ENTERITIS
(II-IV° WHO)
Grade II°
Grade III°
Grade IV°
16/130 (12%)
13/130 (10%)
3/130 (2%)
0/130 (0%)
LIVER TOXICITY (WHO)
Grade II°
Grade III°
Grade IV°
10/130 (8%)
9/130 (7%)
1/130 (1%)
0/130 (0%)
Fever during
GO infusion
58/130 (45%)
VOD
0/130
Other
Encephalitis 1/130
Table 2B: Italian experience with go (3mg/Sqm) +FLAI. A) Hematologic and B) Extrahemoltologic toxicities (Candoni 2014).
Thus, the final results of this trial, even if phase 2, single-arm, not randomized, confirmed the safety of low dose of GO (3 mg/ sqm) when added to induction therapy and suggested that FLAI-GO is an effective induction regimen for AML patients younger than 65 years, with a high complete response rate (ORR 87%), favourable safety profile, low DDI, and sustained RFS, allowing consolidation therapy with SCT early and in a high proportion of cases (62%) [13]. Moreover, the results of this study support, in accordance with the results of the trials previous summarized, the reassessment of GOcontaining combination as front-line AML therapy.
Discussion and Conclusion
Gemtuzumab-ozogamicin, (GO) was the first example of antibody targeted therapy developed for AML. According to the opinion of the majority of AML experts GO was prematurely withdrawn from US and European markets, and its role in induction chemotherapy should be re-evaluated [5,26-29]. Overall, in most randomized trials where a lower dose of GO (3-6 mg/sqm) was combined with intensive induction therapy in young adults and pediatric patients with AML, a significant survival benefit (even without improving the initial response rate) particularly in the specific subgroups of AML with favourable and intermediate risk cytogenetic, was achieved [4,5]. The differences between the positive GO trials and the SWOG S0106 trial, that caused the withdrawn of the drug, can be clarified. Firstly, in this trial, the dose of daunorubicin in the GO arm was lower compared the control arm (45 mg/sqm vs. 60 mg/sqm) and this may negatively affected the ORR and the positive effect of GO. Secondly, in the control arm of this trial the mortality was unusually low (1%) while the mortality in the GO arm (6%) was in line with other conventional induction schemes. Thirdly, in the four favourable studies GO was delivered as a fractionated and/or lower doses, resulting in less hematologic and extra-hematologic toxicity.
Unfortunately, the favourable data regarding GO combined with induction chemotherapy in young adults with AML, have been published after the withdrawn of GO and, for this reason, the FDA decision should be revised and changed.
Clearly, based on the recent published studies, some aspects of GO target therapy should be reviewed, in order to identify the best candidate who could benefit from this drug. Particularly, some key items regarding GO therapy include: the administration schedule and dose of GO when combined with chemotherapy, the interactions between GO and MDR phenotype, the relationship between GO efficacy and the amount of CD33 expression and the concerns on safety profile of GO.
Considering the timing and administration schedule of GO, some studies indicated that surface CD33 levels returned to pre-treatment levels within 72 hours after anti-CD33 antibody administration, despite internalization and modulation. This suggests that repeated administrations of lower, but saturating, doses of GO may enhance intracellular accumulation of calicheamicin over the initial administration schedule of 9 mg/sqm every 2 weeks [4,5,30]. In addition, data from trials in which GO has been used at lower doses suggested that low doses of GO were able to increase the intensity of induction or salvage therapy without increasing its toxicity [4,5,19,21,30,31]. In fact, in most of the recent phase 3 trials, there were no significant differences with regard to non-hematologic toxicities between the GO arm and the control arm, when GO was added to induction chemotherapy [4,19,21]. The same results were observed in the Italian study with an extra-hematologic toxicity in line with a conventional induction chemotherapy scheme [12,13]. In conclusion, all recent data support the use of lower (3 mg/sqm), and repeated doses, of GO in combination with chemotherapy.
It is well established that ABC transporter activity, in particular mediated by P-glycoprotein (PGP), predicts for therapeutic failure of standard induction therapy with persistence of AML marrow blasts, failure to achieve CR or reduced in vitro drug-induced apoptosis. By analogy to other chemotherapeutic agents (such as antracyclines) the cytotoxicity of GO is influenced by the expression of the multidrug resistance (MDR) phenotype of AML blasts [32]. Particularly, PGP over-expression leads to expulsion of calicheamicin resulting in clinical resistance to GO [33-35]. In detail, after the lysosome hydrolysis of GO, the calicheamicin is detached and intracellularly released but, in MDR positive blast cells, it can be pumped out through PGP. Obviously, this susceptibility to cellular drug efflux may significantly limit the therapeutic efficacy of GO, especially for the treatment of relapsed or refractory AML where PGP is often overexpressed [33-35]. Recently, Walter et al, demonstrated that patients responding to GO had significant lower PGP activity and higher CD33 expression than non responsive cases [36,37].
The relationship between amount of CD33 expression and GO efficacy remains uncertain. In the majority of trials, patients were not selected according to the CD33 expression status. Some studies did not find a correlation between CD33 expression levels and response to GO [38]. Particularly, in the AMLMRC15 trial and in the ALFAPATIENT 0701 trial the CD33 expression status (> 20% classified as positive) did not seem to have a predictive value for survival [19,21]. However, van der Velden et al found that the effect of GO on marrow blast cells was related with the expression of CD33 in blood blast cells [39]. Particularly, in case of high peripheral blast count, GO may be lost in the circulation before it reaches the bone marrow [39]. In clinical practice, this suggest that GO might be made more effective by the reduction of CD33 positive blast cells in peripheral blood by chemotherapy before the GO administration [32, 39].
Undoubtedly, an example of the excellent sensitivity to GO is the acute promyelocytic leukemia (APL) in which the efficacy of GO is well established both in the front-line and in relapse and may be partially explained with the well know over-expression of the CD33 antigen in APL blast cells coupled with their low drug efflux activity (the low PGP expression is a common finding in APL) [40,41].
The toxicity of GO is still of concern and a peculiar side effect, that was reported very early, is liver veno-occlusive disease (VOD) [14,42]. Symptoms of GO-associated VOD can include: rapid weight gain (due to fluid retention), right upper quadrant pain, hepatomegaly, ascites, elevations in bilirubin and/or liver enzymes and portal hypertension. Developing a median of 10–14 days following GO treatment, VOD occurs more likely when the drug is given at doses higher than 6 mg/sqm or when it is combined with a hepatotoxic agent (e.g. thioguanin). Patients who had received GO after allo SCT and those who had received allo SCT (within 3–4 months) following GO, were at a higher risk of VOD (15-22%) than patients who had not been transplanted (1%) [42,43]. The etiology of GO-related VOD remains unclear although proposed mechanisms include exposure to unconjugated calicheamicin-γ1 in the circulation, nonspecific uptake of GO by CD33 + Kupffer cells or CD33-mediated uptake of GO by one or more of the cell populations in the liver that expressed CD33 (some data suggest that CD33 is also found on hepatocytes). Indeed, in the induction setting, when lower doses of GO were combined with chemotherapy, the occurrence of VOD and hepatotoxicity were very low, as reported in both ALFA-0701 and MRC15 trials and also confirmed in our experience [13,19,21]. Thus, the hepatotoxicity of GO (and specifically VOD), is most common when GO is given at higher doses (9 mg/sqm) or in combination with hepatotoxic agents and when using GO within a few months before or after allo SCT.
In summary, GO was withdrawn from the US and European markets in 2010 due to post marketing concerns about the drug safety and of lack of efficacy (SWOG-S0106 trial). However, after withdrawal from the market, several large, well-controlled, randomized clinical trials, combining lower and fractionated doses of GO to standard first-line chemotherapy, showed better tolerability and clear efficacy with significant improvement of EFS and OS, particularly in AML with favorable and intermediate-risk cytogenetics, leading to renewed interest in this drug. It is worthy of note that the ALFA 0701 trial reported a median EFS of 19,6 months for GO plus chemotherapy combination vs. 11,9 months for chemotherapy alone, while median OS was 34 months vs. 19,2 months, respectively. In addition, the metanalysis of available data suggest that, using a fractionated dosing regimen of GO (3 mg/sqm per administration) the adverse events were substantially lower than those reported with the single-dose infusion of the drug (9 mg/sqm, one shot) [4, 14, 44]. These results cannot be ignored in an era of tailored and targeted therapy. Thus, even if there is no precedent for a drug to be withdrawn and then successfully reintroduced, the regulatory approval should be revised leading to restore GO as a treatment option at least for some subsets of AML.
However, in clinical practice some efforts are needed to identify the AML patients that most likely benefit from such agent. In our opinion, taking into account the new available data, the best AML candidate to receive GO therapy should have the following characteristics: age less than 60 years, no hepatic diseases, de novo AML, first induction phase, favorable or intermediate cytogenetic risk, no PGP over-expression (no MDR phenotype), and expression of CD33 on blast cells over 20%. The preferred schedule of GO, in addition to induction chemotherapy, should include lower doses and repeated administrations (e.g. 3 mg/sqm days 1,4,7) to avoid toxicity without affecting efficacy as proposed by ALFA group. The chemotherapy in association of GO should include cytarabine plus daunorubicin based regimens (e.g DA, DAE) or FLAI scheme which includes drugs not MDR related such as fludarabine and a more potent and less PGP sensitive anthracycline (idarubicin) (Table 3).
PATIENT
CHARACTERISTICS
-AGE < 60 years.
-No Hepatic Diseases.
-PS < 2
AML
CHARACTERISTICS
-De Novo AML.
-LOW or INTERMEDIATE RISK.
-PGP NOT OVEREXPRESSED.
-(CD33 EXPRESSION > 20%).
GO THERAPY
CHARACTERISTICS
- Combine GO with anthracyclines and
cytarabine based induction chemotherapy
(e.g. DA, DAE, FLAI).
-LOW DOSE of GO (3 mg/sqm).
-REPEAT for 2-3 doses (ALFA0701 trial)
(e.g. GO 3-3-3 regimen, day 1,4,7).
-If hyperleukocytosis, place the first dose of
GO after leukemia debulking (between 2°
and 6° day from start chemotherapy).
Table 3: An attempt at identifying the characteristics of the best candidate to receive induction therapy with GO plus chemotherapy.
References
- Estey E. Why Is Progress in Acute Myeloid Leukemia So Slow? Semin Hematol. 2015; 52: 243-248.
- Freireich EJ, Wiernik PH, Steensma DP. The leukemias: a half-century of discovery. J Clin Oncol. 2014; 32: 3463-3469.
- Loke J, Khan JN, Wilson JS, Craddock C, Wheatley K. Mylotarg has potent anti-leukaemic effect: a systematic review and meta-analysis of anti-CD33 antibody treatment in acute myeloid leukaemia. Ann Hematol. 2015; 94: 361-373.
- Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014; 15: 986-996.
- Laszlo GS, Estey EH, Walter RB. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev. 2014; 28: 143-153.
- Stasi R, Evangelista ML, Buccisano F, Venditti A, Amadori S. Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer Treat Rev. 2008; 34: 49-60.
- Pagano L, Fianchi L, Caira M, Rutella S, Leone G. The role of Gemtuzumab Ozogamicin in the treatment of acute myeloid leukemia patients. Oncogene. 2007; 26: 3679-3690.
- Giles F. Estey E, O’Brien S. Gemtuzumab Ozogamicin in the Treatment of Acute Myeloid Leukemia. Cancer. 14:1436–1443.
- Larson RA, Boogaerts M, Estey E, Karanes C, Stadtmauer EA, Sievers EL, et al. Mylotarg Study Group. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin). Leukemia 2002; 16: 1627-1636.
- Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001; 7: 1490-1496.
- Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001, 19: 3244–3354.
- Candoni A, Martinelli G, Toffoletti E, Chiarvesio A, Tiribelli M, Malagola M, et al. Gemtuzumab-ozogamicin in combination with fludarabine, cytarabine, idarubicin (FLAI-GO) as induction therapy in CD33-positive AML patients younger than 65 years. Leuk Res. 2008; 32: 1800-1808.
- Candoni A, Martinelli G, Gherlinzoni F, et al. Low dose Gemtuzumab ozogamicin plus FLAI as induction therapy in CD33-positive AML. Definitive results and long term outcome of a phase III multicenter prospective clinical trial (NCT.00909168). Haematologica. 2014; 99: 33.
- Li X, Xu SN, Qin DB, Tan Y, Gong Q, Chen JP. Effect of adding gemtuzumab ozogamicin to induction chemotherapy for newly diagnosed acute myeloid leukemia: a meta-analysis of prospective randomized phase III trials. Annals of Oncology. 2014; 25: 455–461.
- Kell WJ, Burnett AK, Chopra R, Yin JA, Clark RE, Rohatiner A, et al. A feasibility study of simultaneous administration of gemtuzumab ozogamicin with intensive chemotherapy in induction and consolidation in younger patients with acute myeloid leukemia. Blood. 2003; 102: 4277-4283.
- Tsimberidou AM, Giles FJ, Estey E, O'Brien S, Keating MJ, Kantarjian HM. The role of gemtuzumab ozogamicin in acute leukaemia therapy. Br J Haematol. 2006; 132: 398-409.
- Dowell JA, J Korth-Bradley, H Liu, King SP, Berger MS. Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse J. Clin. Pharmacol. 2001; 41; 1206-1214.
- Ricart AD. Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin Cancer Res. 2011; 17: 6417-6427.
- Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011; 29: 369-377.
- Delaunay J, Recher C, Pigneux A. Addition of Gemtuzumab Ozogamycin to Chemotherapy Improves Event-Free Survival but Not Overall Survival of AML Patients with Intermediate Cytogenetics Not Eligible for Allogeneic Transplantation. Results of the GOELAMS AML 2006 IR Study. Presented at ASH 2011. Blood. 2011; 118: 37-38.
- Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012; 379: 1508-1516.
- Burnett AK, Hills RK, Hunter AE, Milligan D, Kell WJ, Wheatley, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013; 27: 75-81.
- Cooper TM, Franklin J, Gerbing RB, Alonzo TA, Hurwitz C, Raimondi SC, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children's Oncology Group. Cancer. 2012; 118: 761-769.
- Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014; 32: 3021-3032.
- Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, et al. A phase III study of gemtuzumab ozogamicin during induction and post-consolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013; 121: 4854-4860.
- Estey E. Treatment of AML: resurrection for gemtuzumab ozogamicin? Lancet. 2012; 379: 1468-1469.
- Foran JM. Gemtuzumab: time to bring back on the market? Clin Adv Hematol Oncol. 2012; 10: 326-327.
- Rowe JM, Löwenberg B. Gemtuzumab ozogamicin in acute myeloid leukemia: a remarkable saga about an active drug. Blood. 2013; 121: 4838-4841.
- Ravandi F, Estey EH, Appelbaum FR, Lo-Coco F, Schiffer CA, Larson RA, et al. Gemtuzumab ozogamicin: time to resurrect? J Clin Oncol. 2012; 30: 3921-3923.
- Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N, Bouabdallah R . High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007; 21: 66-71.
- Pilorge S, Rigadeau S, Rabian F, Sarkozy C, Taksin AL, Farhat H, et al. Fractionated gemtuzumab ozogamicin and standard dose cytarabine produced prolonged second remissions in patients over the age of 55 years with acute myeloid leukemia in late firs relapse. Am J Hemat. 2014; 89: 399-402.
- Linenberger ML, Hong T, Flowers D, Sievers EL, Gooley TA, Bennett JM, et al. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood. 2001; 98: 988-994.
- McKoy JM, Angelotta C, Bennett CL, Tallman MS, Wadleigh M, Evens AM, et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res. 2007; 31: 599–604.
- Matsui H, Takeshita A, Naito K, Shinjo K, Shigeno K, Maekawa M, et al. Reduced effect of gemtuzumab ozogamicin (CMA-676) on P-glycoprotein and/or CD34-positive leukemia cells and its restoration by multidrug resistance modifiers. Leukemia 2002; 16: 813–819.
- Naito K, Takeshita A, Shigeno K. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab ozogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia. 2003; 98: 10.
- Walter RB, Gooley TA, van der Velden VH, Loken MR, van Dongen JJ, Flowers DA, et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007; 109: 4168-4170.
- Walter RB, Raden BW, Hong TC, Flowers DA, Bernstein ID, Linenberger ML. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood. 2003; 102: 1466-1473.
- Jedema I, Barge RM, van der Velden VH, Nijmeijer BA, van Dongen JJ, Willemze R, et al. Internalization and cell cycle-dependent killing of leukemic cells by Gemtuzumab Ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia. 2004; 18: 316-325.
- van der Velden VH, Boeckx N, Jedema I, te Marvelde JG, Hoogeveen PG, Boogaerts M, et al. High CD33-antigen loads in peripheral blood limit the efficacy of gemtuzumab ozogamicin (Mylotarg) treatment in acute myeloid leukemia patients. Leukemia. 2004; 18: 983-988.
- Breccia M, Lo-Coco F. Gemtuzumab ozogamicin for the treatment of acute promyelocytic leukemia: mechanisms of action and resistance, safety and efficacy. Expert Opin Biol Ther. 2011; 11: 225-234.
- Ravandi F, Estey E, Jones D, Faderl S, O'Brien S, Fiorentino J, Pierce S. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009; 27: 504-510.
- Rajvanshi P, Shulman HM, Sievers EL, McDonald GB. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood. 2002; 99: 2310-2314.
- Tallman MS, McDonald GB, DeLeve LD, Baer MR, Cook MN, Graepel GJ, et al. Incidence of sinusoidal obstruction syndrome following Mylotarg (gemtuzumab ozogamicin): a prospective observational study of 482 patients in routine clinical practice. Int J Hematol. 2013; 97: 456-464.
- Sylvie Castaigne. Why is it so difficult to use gemtuzumab ozogamicin? Blood. 2013; 121: 4813-4814.