
Research Article
Ann Hematol Oncol. 2015; 2(8): 1056.
The Combination of Fludarabine + Citarabine + Idarubicin (Flai), Followed by Post-Remission Maintenance, is a Safe and Effective Treatment for Elderly Patients with a ml or High Risk Mds
Crisà E, Cerrano M, Gatti T, Giai V, Aguzzi C, Boccadoro M and Ferrero D*
Department of Molecular Biotechnologies and Health Sciences, Hematology Section, University of Turin, Italy
*Corresponding author: Dario Ferrero, Hematology Section, University of Turin, Via Genova 3, 10126, Torino, Italy
Received: July 21, 2015; Accepted: November 15, 2015; Published: November 17, 2015
Abstract
Acute myeloid leukemia (AML) in the elderly has a dismal outcome due to both patients and disease characteristics. Fludarabine, high dose ARA-C and idarubicin combination (FLAI) has been reported to be effective in poor prognosis AML. Here we present our experience with FLAI induction regimen in patients over 60 with AML or high risk myelodysplasia (MDS) followed by post-CR maintenance (low-dose chemotherapy plus retinoids and vitamin D as differentiating agents) in patients unfit for allogeneic transplantation. Median age at diagnosis was 66 (60-78). Half (47%) of the patients were at poor prognosis for secondary/therapy related AML (26%) or MDS (21%). Thirty-seven percent of patients had an adverse karyotype. Twenty-two patients received FLAI at reduced intensity for reduced performance status.
CR rate was 59%, with 5% failing because of induction death and 36% because of resistant disease. CR achievement was not affected by age or dose reduction, only by cytogenetics.
The death rate in CR was 6% and the relapse rate was 60%, leading to 5-year overall and disease-free survival of 23 and 27% (median 14 and 20 months), respectively. Survival was negatively affected by adverse cytogenetics and by MDS diagnosis as compared to AML.
After CR, 7 patients underwent allogeneic transplantation (median survival not reached) and 24 received a maintenance treatment (median survival 47 months).
A good CR quality induced by FLAI and a benefit of the maintenance treatment may explain these fair long-term results. Our encouraging results should be confirmed in a randomized trial.
Keywords: FLAI; AML; MDS; Maintenance treatment
Introduction
Median age at diagnosis of acute myeloid leukemia (AML) has now reached 70 years old. Older patients have a dismal prognosis due to unfavorable prognostic factors, both disease related (i.e. AML secondary to myelodysplastic syndrome (MDS), higher incidence of complex karyotype) and patient related (comorbidities and performance status) [1].
The treatment choice can go from intensive chemotherapy to supportive care and often stays in the middle. Novel drugs have been studied but long term survival with low intensity treatment is still quite short.
It has been shown how the survival depends on the remission achievement, therefore current strategy is to choose the treatment aiming to achieve a complete response whenever the performance status allow it and regardless the age.
The purine analog fludarabine in addition to cytosine-arabinoside (ARA-C) increases the accumulation of ARA-C-5’-triphosphate, which is responsible for the cytotoxic effect in leukemic blasts, resulting in improved cell kill [2].
A number of phase 2 studies have been published on the combination of fludarabine, ARA-C and idarubicin to treat relapsed or refractory AML, high-risk MDS and AML in the elderly [3–12]. Remission rates vary from 50% to 60% and the median duration of remission from 4 to 12 months.
Some randomized studies comparing FLAG or FLAG-IDA to standard 3+7 regimes in young AML patients showed at least comparable results in terms of complete remission (CR) rates [13– 15].
Incidence of treatment-related deaths ranged from 7 to 30%, higher in the oldest studies and decreasing in the last decade.
In this study we present the data on our experience with FLAI as induction regimen, for elderly patients with AML or high risk MDS. We also re-evaluated a previously described post-CR maintenance treatment [16] in patients achieving CR with FLAI regimen and unable to undergo allogeneic stem cell transplantation (allo-HSCT).
Patients and Methods
Since 2002 we treated with FLAI induction therapy 61 patients aged over 60 years old, diagnosed with high risk MDS or AML, excluding APL. Surviving patients were censored at 30st June 2015.
The treatment schedule was fludarabine 30 mg/mq plus cytarabine 2g/m² days 1 to 5 and idarubicin 10 mg/m² on days 1, 3, 5. Patients with serious comorbidities and/or reduced performance status received the same schedule with a reduction of 30% in the idarubicin and cytarabine doses and 20% in fludarabine dose. Following completion of the induction schedule, responding patients could proceed to consolidation with another course of FLAI, reduced to 4 days. Few patients received a further consolidation course.
Subsequently, fit patients with an available donor underwent allo- HSCT. The other patients were scheduled to receive a maintenance treatment based on the alternation of low-dose cytarabine plus 6-mercaptopurine and 6-thioguanine together with differentiating agents (retinoid acid and dihydroxylate vitamin D3) [16].
Patients who failed to achieve CR after the FLAI regimen received a different intensive chemotherapy course (clofarabine + ARA-C N =4, idarubicin + ARA-C+ etoposide N=1) or palliative treatments. One patient with a very good partial remission received a second induction treatment with FLAI regimen.
CR was defined by marrow blasts <5% with normal cellularity and morphology plus blood counts with haemoglobin >10 g/dl, neutrophils >1.5x109/l, platelets >100x109/l. This slight degree of peripheral blood cytopenia was still considered compatible with a CR in the presence of normal marrow cellularity and blast proportion, since patients in the maintenance group were in continuous treatment and did not stop cytotoxic drugs for more than 3 weeks.
Relapse was defined by the reappearance of a marrow blast percentage higher than 5% or, for MDS patients, of treatment unrelated cytopenia.
Statistical methods
Demographic and clinical covariates were compared using Pearson chi-square test. Overall survival (OS) was calculated from diagnosis until death or last follow up, and disease free survival (DFS) was assessed from remission until relapse, death for any cause or last follow up. Kaplan-Meier method was used to estimate the probabilities of OS and DFS. Log-rank test was used to compare subgroups of patients in terms of OS or DFS. Univariate and multivariate analyses were performed using the Cox model. All statistical analyses were conducted using SPSS.
Results
Median follow up for censored patients was 51 months (range 6 to 140 months). For main patients characteristics see Table 1.
Sex
M
37 (61%)
F
24 (39%)
Age at diagnosis (years)a
66 (60-78)
over 65
33 (57%)
over 70
8 (13%)
Cytogenetic risk groupsb
Good
1 (2%)
intermediate
30 (61%)
poor
19 (37%)
not determinate
11
Diagnosis
AML
48 (79%)
de novo AML
42 (87%)
secondary to MDS
15 (31%)
therapy related
1 (2%)
MDS/CMML
13 (21%)
therapy related
3 (23%)
Consolidation chemotherapy (cycles)a
1 (0-2)
autologous transplantation
3
Allogeneic transplantation at any time
8
Maintenance treatment
24
amedian (range).
bCytogenetic risk-based subgroups according to MRC Refined [18]
Table 1: Description of 61 evaluable patients.
Median age at diagnosis was 66 (range 60 to 78 years) and 61% of patients were male.
Seventy-nine percent of patients were diagnosed with AML, in 31% of them secondary to MDS, and 21% with high risk MDS, including chronic myelo-monocitic leukemia. Overall, 4 patients only (7%) had a therapy- related disease.
Thirty-seven percent of patients had an adverse cytogenetics and 1 patient only had a good prognosis karyotype (inversion 16). Twenty-two patients received FLAI regimen at reduced intensity.
Thirty-five patients achieved a CR after the first FLAI course, 1 obtained the CR with a second course of FLAI for a total number of 36 (59% of the entire population) with 5% failing because of induction death (N=3) and 36% because of resistant disease (N=22).
Dose reduction did not affect CR achievement, as AML vs. MDS diagnosis, age or sex. By contrast patients with high risk cytogenetics had a significantly lower CR rate, p=0,017.
Out of the 22 primary refractory patients, 2 patients achieved CR with salvage chemotherapy: 1 of them underwent allo-HSCT and 1 received maintenance treatment until relapse.
Regarding post remission treatment after FLAI induced CR, 7 patients underwent allo-HSCT and 24 patients received a maintenance treatment. One patient with favorable cytogenetic received 3 consolidation courses with intermediate/high dose ARA-C; four had a very early disease relapse within 3 months from 1st CR, before the maintenance start.
The death rate in CR was 6% (1 patient for cardiovascular event and 1 transplant- related) and the relapse rate was 60%, leading to 5-year DFS of 27% (median 20 months) (Figure 1).
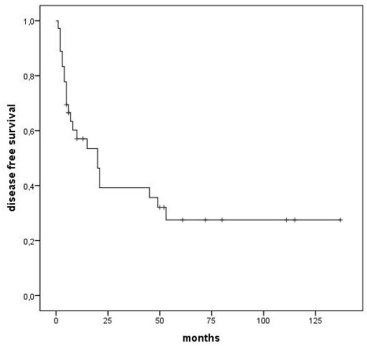
Figure 1: Disease free survival, all patients.
As expected, 71% of patients with MDS and 100% of patients with poor prognosis karyotype relapsed.
OS at 5 years was 23% for the whole cohort (median 14 months) (Figure 2). Primary refractory patients had the worst outcome with no patients surviving at 5 years and a median OS of 9 months.
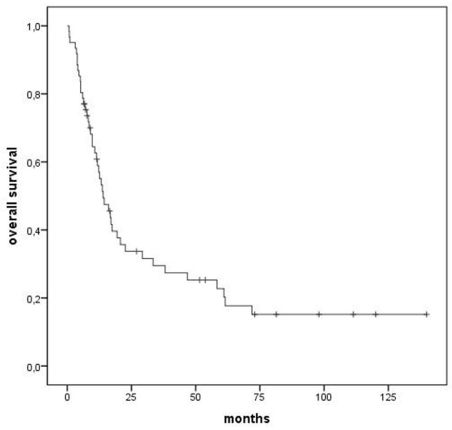
Figure 2: Overall survival, all patients.
Survival was negatively affected by adverse cytogenetics (median 13 months vs. 19 months, p=0,030) (Figure 3) and by MDS diagnosis as compared to AML (median 8 months vs. 17 months), both prognostic factors confirmed at multivariate analysis too (p=0,024 and p=0,001, respectively) (Figure 4).
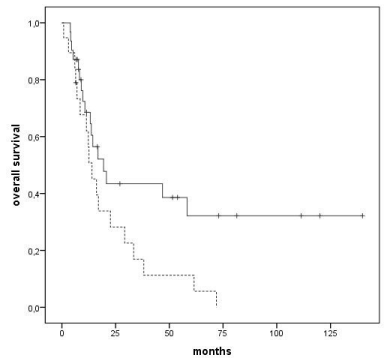
Figure 3: Overall survival according to cytogenetic risk: good prognosis
(continuous line), intermediate (dotted line), adverse (dashed line), p=0,030.
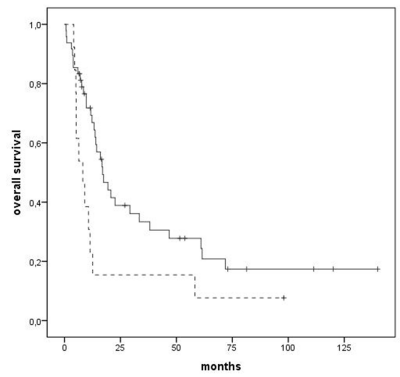
Figure 4: Overall survival according to diagnosis: AML (continuous line) vs.
MDS (dashed line), p=0,024.
There were no differences in survival according to age groups (60- 65 or 65-70 or >70).
Median survival for transplanted patients is still not reached and for patients who received maintenance therapy is 47 months.
Toxicity
FLAI regimen induced grade 4 hematology toxicity in all patients, as expected for an intensive anti- AML regimen, however, only 3 induction death, for infections, were recorded. Other extrahematological toxicities were generally mild, without grade 3-4 events.
Discussion
In this paper we retrospectively report our experience with FLAI regimen in AML and high risk MDS patients aged over 60, a main poor- prognosis feature. The combination of fludarabine + high dose ARA-C +/- idarubicin has been previously reported as an effective regimen for poor prognosis AML (particularly in relapsed/ refractory disease) and as front-line therapy in young patients [3-15]. We chose FLAI regimen, with mild dosage reductions in elderly/poor performance patients, in the attempt to improve the generally poor results of standard AML treatments above the age of 60. We also adopted a post-CR maintenance treatment with low dose chemotherapy+ retinoids and dihydroxylated vitamin D3 as differentiating agents, aiming to prolong the short CR duration in old AML/MDS patients unsuitable to allo-HSCT. We have previously reported the efficacy of this approach in a group of poor-risk AML/ MDS patients of various age and treated with different induction regimens [16]. Here we re-evaluated the patients more than 60 year old who had received the maintenance treatment after FLAI- induced CR.
Besides the age over 60, almost half (47%) of our patients had a poor prognosis diagnosis such as secondary therapy related AML (26%) or high risk MDS /CMML (21%). Moreover, 37% of patients with an available cytogenetic test had a poor prognosis karyotype and 1 patient only had a favorable cytogenetic abnormality (inv16).
By considering the above described poor- prognosis features, the CR rate was fair (64% for AML and 54% for MDS patients) in the range of best results obtained in patients over 60 years old. Interestingly, the induction death rate was 5% only, lower than usually reported for patients above the age of 60. This is in agreement to the generally good tolerability of our treatment.
As expected, patients who failed to achieve CR with FLAI regimen had a poor outcome: only a few of them were fit enough to receive salvage chemotherapy and only 2 achieved CR with a combination of clofarabine + ARA-C. However, the OS of the whole group of patients (median 14 months; 23% alive at 5 years from diagnosis) was better than usually observed in patients with similar poor prognostic features. This result is due to the fair DFS (27% at 5 years) of patients who achieved CR with FLAI regimen, despite the low proportion of patients undergoing allo-HSCT. In particular, among patients who achieved a CR lasting at least 2-3 months, so to either undergo allo- HCST or to start the maintenance treatment, the median OS was almost 4 years (47 months).
A benefit of the maintenance treatment and a good CR quality induced by FLAI regimen may explain the fair long-term results obtained with our patients. Nonetheless the outcome of the patients with unfavorable karyotype remained poor and it was worse in MDS than in AML patients, although the number of MDS patients was low.
Of note, no difference in survival was observed according to age groups or dose intensity, this suggesting that treating patients according to fitness status and not to the biological age is effective.
Although the results of our approach for old / poor risk AML patients look encouraging, it should be prospectively compared to other protocols that, although using standard- dose ARA-C have reported very high rate of CR in elderly patients [17].
References
- Ossenkoppele G, Löwenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015; 125: 767–774.
- Gandhi V, Estey E, Keating MJ, Plunkett W. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J. Clin. Oncol. 1993; 11: 116–124.
- Russo D, Pricolo G, Michieli M, Michelutti A, Raspadori D, Bertone A, et al. Fludarabine, arabinosyl cytosine and idarubicin (FLAI) for remission induction in poor-risk acute myeloid leukemia. Informa UK Ltd UK; 2001; 40: 335-343.
- Parker JE, Pagliuca A, Mijovic A, Cullis JO, Czepulkowski B, Rassam SM, et al. Fludarabine, cytarabine, G-CSF and idarubicin (FLAG-IDA) for the treatment of poor-risk myelodysplastic syndromes and acute myeloid leukaemia. Br. J. Haematol. 1997; 99: 939–944.
- Nokes TJ, Johnson S, Harvey D, Goldstone AH. FLAG is a useful regimen for poor prognosis adult myeloid leukaemias and myelodysplastic syndromes. Leuk. Lymphoma. 1997; 27: 93–101.
- Visani G, Tosi P, Zinzani PL, Manfroi S, Ottaviani E, Testoni N, et al. FLAG (fludarabine + high-dose cytarabine + G-CSF): an effective and tolerable protocol for the treatment of “poor risk” acute myeloid leukemias. Leukemia. 1994; 8: 1842–1846.
- Ferrara F, Leoni F, Pinto A, Mirto S, Morra E, Zagonel V, et al. Fludarabine, cytarabine, and granulocyte-colony stimulating factor for the treatment of high risk myelodysplastic syndromes. Cancer. 1999; 86: 2006–2013.
- Montillo M, Mirto S, Petti MC, Latagliata R, Magrin S, Pinto A, et al. Fludarabine, cytarabine, and G-CSF (FLAG) for the treatment of poor risk acute myeloid leukemia. Am. J. Hematol. 1998; 58: 105–109.
- Steinmetz HT, Schulz A, Staib P, Scheid C, Glasmacher A, Neufang A, et al. Phase-II trial of idarubicin, fludarabine, cytosine arabinoside, and filgrastim (Ida-FLAG) for treatment of refractory, relapsed, and secondary AML. Ann. Hematol. 1999; 78: 418–425.
- Jackson G, Taylor P, Smith GM, Marcus R, Smith A, Chu P, et al. A multicentre, open, non-comparative phase II study of a combination of fludarabine phosphate, cytarabine and granulocyte colony-stimulating factor in relapsed and refractory acute myeloid leukaemia and de novo refractory anaemia with excess of blasts in . Br. J. Haematol. 2001; 112: 127–137.
- Virchis A, Koh M, Rankin P, Mehta A, Potter M, Hoffbrand AV, et al. Fludarabine, cytosine arabinoside, granulocyte-colony stimulating factor with or without idarubicin in the treatment of high risk acute leukaemia or myelodysplastic syndromes. Br. J. Haematol. 2004; 124: 26–32.
- Kim I, Koh Y, Yoon S-S, Park S, Kim BK, Kim D-Y, et al. Fludarabine, cytarabine, and attenuated-dose idarubicin (m-FLAI) combination therapy for elderly acute myeloid leukemia patients. Am. J. Hematol. 2013;88: 10-15.
- Milligan DW, Wheatley K, Littlewood T, Craig JIO, Burnett AK. Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood. 2006; 107: 4614–422.
- Burnett AK, Russell NH, Hills RK, Hunter AE, Kjeldsen L, Yin J, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J. Clin. Oncol. 2013; 31: 3360–3368.
- Russo D, Malagola M, de Vivo A, Fiacchini M, Martinelli G, Piccaluga PP, et al. Multicentre phase III trial on fludarabine, cytarabine (Ara-C), and idarubicin versus idarubicin, Ara-C and etoposide for induction treatment of younger, newly diagnosed acute myeloid leukaemia patients. Br. J. Haematol. 2005; 131: 172–179.
- Ferrero D, Crisà E, Marmont F, Audisio E, Frairia C, Giai V, et al. Survival improvement of poor-prognosis AML/MDS patients by maintenance treatment with low-dose chemotherapy and differentiating agents. Ann. Hematol. 2014; 93: 1391–1400.
- Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N. Engl. J. Med. 2009; 361: 1235–1248.
- Grimwade D. The changing paradigm of prognostic factors in acute myeloid leukaemia. Best Pract Res Clin Haematol. 2012; 25: 419–425.
Citation: Crisà E, Cerrano M, Gatti T, Giai V, Aguzzi C, Boccadoro M, et al. The Combination of Fludarabine + Citarabine + Idarubicin (Flai), Followed by Post-Remission Maintenance, is a Safe and Effective Treatment for Elderly Patients with a ml or High Risk Mds. Ann Hematol Oncol. 2015; 2(8): 1056. ISSN : 2375-7965