
Case Report
Ann Hematol Oncol. 2015; 2(8): 1058.
Granulocytic Sarcoma Involving the Gynecologic Tract: One Case Report and Review of the Literature
Quintela A¹, Ducreux S¹, Lequeu H¹, Chassagne C², Plesa A¹, Le Jeune C¹ and Thomas X¹*
¹Department of Hematology, Lyon-Sud Hospital, France
²Centre Léon Bérard, Laboratory of Anatomopathology, France
*Corresponding author: Xavier Thomas, Department of Hematology, Lyon-Sud Hospital, Bat. 1G, 165 chemin du Grand Revoyet, 69495 Pierre Bénite Cedex, France
Received: August 24, 2015; Accepted: November 10, 2015; Published: November 15, 2015
Abstract
Granulocytic sarcoma is an extramedullary tumor associated with acute myeloid leukemia (AML). It is rarely seen in the female tract. We report an unusual case of granulocytic sarcoma of the uterus in an AML patient who relapses after 14 years of first complete remission. A mixed-lineage leukemia (MLL) gene rearrangement supports the association of that malignancy with prior chemotherapy. Second line therapy consisted in chemotherapy, radiation, and allogeneic stem cell transplantation.
Keywords: Acute myeloid leukemia; Granulocytic sarcoma; Prognosis; Treatment; Gynecologic tract
Introduction
Myeloid sarcomas are rare extramedullary tumors of immature myeloid cells. They comprise two major subtypes: granulocytic sarcomas composed of granulocytic precursors at various stages of differentiation and monoblastic sarcoma which consist of monoblasts and immature monocytes [1]. In the largest published series of myeloid sarcomas, 50% were of granulocytic type, 43.5% either monoblastic or myelomonoblastic, and 6.5% corresponded to different histotypes [2]. The lesion was first described by Burns in 1811 [3]. It was named ‘chloroma’ because it displays a greenish colour due to the presence of myeloperoxidase (or verdoperoxydase) [4] and was then found to be associated with acute myeloid leukemia (AML) [5,6]. The preferred term of ‘granulocytic sarcoma’ was coined later [7]. Myeloid sarcoma resembles a solid tumor and should not be confused with diffuse extramedullary leukemic infiltrates. It is most commonly diagnosed as part of the systemic manifestations of AML, and develops in 2-8% of patients with AML [8,9]. On rare occasions, it may also be the presenting symptom. Myeloid sarcoma may also be the sole manifestation of relapse of previously treated myeloid leukemia. Granulocytic sarcoma may occur at almost any body site. Involvement of the female genital tract is uncommon [10]. The most commonly involved organs are the ovary, the uterine cervix and the uterus corpus [11].
We report here one case of granulocytic sarcoma of the uterine cervix with extension to the left parametrium concomitant to bone marrow involvement in one patient treated 14 years earlier for AML.
Case Presentation
A 44-year old woman (gravida 2, para 2) (with a past history of surgery for adenofibroma of the left breast in 1985) presented to our institution in October 2000 with fatigue, dyspnea, gingival hemorrhage, and menorrhagia. Clinical examination was normal. Blood test displayed white blood cell count at 2.4 x 109/L with 18% neutrophils and 60% circulating blasts, anemia, and thrombocytopenia. Bone marrow aspirate confirmed the diagnosis of FAB M1-AML with medullary infiltration by 40% of blastic cells of which 25% with Auer rods. Cytogenetic studies showed 46, XX [17]/46, XX, add (21) (q21-22) [3]. Molecular analyses were negative for mixed-lineage leukemia (MLL) rearrangement; CEBPa, FLT3, and NPM1 mutations; and WT1 overexpression. After given written informed consent, she was included into the ALFA 9802 trial [12]. Induction chemotherapy regimen consisted of a timed sequential chemotherapy with a first sequence combining daunorubicin (80 mg/ m²/d on days 1-3) and cytarabine (500 mg/m²/d over the same period) and a second sequence, administered after a 4-day free interval, with mitoxantrone (12 mg/m²/d on days 8 and 9) and cytarabine (500 mg/ m²/12h on days 8-10). After complete remission (CR) achievement, she was assigned to consolidation chemotherapy, despite one HLAcompatible sibling, in the absence of risk factors. She received 4 cycles of high-dose cytarabine (3 g/m²/12h on days 1, 3, and 5) followed by 4 additional maintenance courses (daunorubicin, 45 mg/m² on day 1, and cytarabine, 100 mg/m²/12h on days 1-5). During chemotherapy, she received granulocyte-macrophage colony-stimulating factor as priming, and lynestrenol was prescribed once daily as prophylaxis against menorrhagia.
After 14 years without any symptoms, she presented again in January 2015 with lumbar pains and functional and obstructive urinary signs, suggesting symptoms in relationship with kidney stone. Sonographic evaluation of the pelvis revealed left ureteropyelocaliceal dilatation. Urinary tract double-J stent was positioned to facilitate the upper urinary tract drainage. CT and magnetic resonance imaging (MRI) scans performed by February 2015 showed an increase in size of the uterus cervix with a tumor of 4 cm of largest diameter and multifocal heterogeneous low signal intensity lesions from the upper vagina to the left parametrium, which suggested a malignant infiltrative process (Figure 1). A pelvic examination confirmed hypertrophy of the cervix. Histopathological examination of multiple colposcopic biopsies of the uterine cervix revealed an infiltration by proliferative, round, small immature myeloid cells among stromal cells (Figure 2). The immunophenotypic study showed that malignant cells expressed vimentin, CD56, CD117, myeloperoxidase, CD43, and CD34, and confirmed the diagnosis of granulocytic sarcoma. Positron emission tomography (PET) scan imaging performed on March 2015 showed hypermetabolism of the cervical mass contiguous with a large left parametrial mass that displaced the left ureter. This was associated with hypermetabolic tumor infiltration of the left iliac axis and hypermetabolic activity in right iliac, aortico-lumbar and intersaortico cave adenopathies.
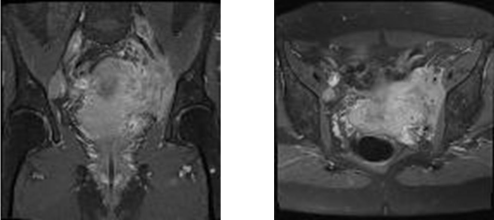
Figure 1: Axial T2 weighted pelvic MRI imaging showing lesions from the
upper vagina to the left parametrium, which suggested a malignant infiltrative
process.
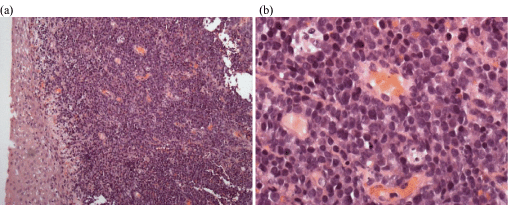
Figure 2: Biopsy from uterine cervix. Diffuse infiltration by immature myeloid
blastic cells; a finding compatible with granulocytic sarcoma: (a) x20; (b) x40.
The patient was hospitalized in the Hematology Department by the end of March 2015. There were no palpable lymph nodes and no hepatosplenomegaly. Laboratory studies revealed the following values: hemoglobin 107 g/L, platelets 228 x 109/L, white blood cells 4.42 x 109/L with 2.65 x 109/L neutrophils and, at that time, no abnormal cells. Bone marrow examination showed an infiltration by 40% of poorly differentiated myeloid blastic cells (Figure 3) (CD34+CD38+/CD123++, CD13+CD33+CD117+) combined with a minor lymphoblastic B CD20-negative sub-clone. Cytogenetics of the bone marrow revealed a normal female karyotype. A duplication of the MLL gene was identified by molecular biological analysis. The patient received re-induction chemotherapy combining idarubicin (10 mg/ m²/day for 3 days) and intermediate-dose cytarabine (1 g/m²/12h for 6 days) [13]. Bone marrow aspirate performed at day 32 confirmed the achievement of a complete medullary response. Post induction chemotherapy PET scan taken on 30th day of treatment showed that the uterine mass had been resolved. Only two hypermetabolic sites persisted in the bladder: one located on the left ureteral meat us and the other one on the left lateral side (Figure 4).
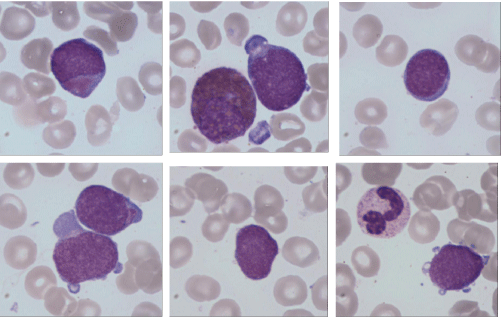
Figure 3: Hematological relapse with poorly differentiated myeloid blastic
cells (MGG x100).
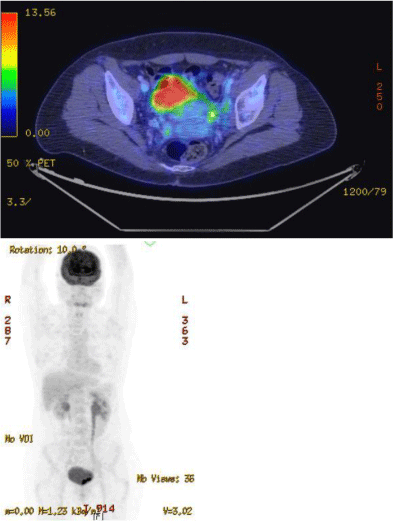
Figure 4: PET scan taken on 30th day of induction therapy showing the
persistence of only two hypermetabolic sites in the bladder.
Consolidation chemotherapy consisted of high-dose cytarabine (3g/m² x 2/day for 3 days). PET scan controlled after recovery from aplasia showed the absence of hypermetabolic sites. The residual density in the left parametrium was consistent with scar tissue. Consolidation chemotherapy was followed by radiotherapy of the pelvis of 24 Gy in 12 fractions of 2 Gy. Allogeneic peripheral blood stem cell transplantation from a HLA-matched sibling donor was performed on August 6th, 2015 after reduced-intensity conditioning regimen (cyclophosphamide 60 mg/Kg, busilvex 3.2 mg/Kg/d for 2 days, and anti-thymocyte globulins for 3 days) following FLAMSA chemotherapy (fludarabine 30 mg/m²/d, amsacrine 100 mg/m²/d, and cytarabine 2 g/m²/d for 4 days) [14].
Discussion
Granulocytic sarcoma estimated incidence is about 2 per million in adults [15]. The mean age at presentation is 47 years [16]. Although asymptomatic disease appears relatively common at autopsy, symptomatic involvement of the female genital tract is a rare presentation of extramedullary AML. Uterus and ovary are the most frequent sites of gynecologic tract involvement. Symptoms of uterus localization are mainly menometrorrhagia, or postcoital, or postmenopausal hemorrhage. The mass often extend to vagina and parametria, and even involved pelvic lymph nodes. Granulocytic sarcoma in the ovary tended to form large rather asymptomatic tumors. Localizations to the vulva and vagina often give physical findings suggesting Bartholin cysts. Granulocytic sarcomas have been rarely described as an initial presentation of AML and are mostly encountered in the course of AML as extramedullary relapse. It can precede or concur with bone marrow blastic infiltration. Diagnosis is often delayed, and prognosis almost always ominous evolving into refractory AML. Most patients who did not have manifest AML at presentation generally developed leukemia by few months. The average interval was estimated between 7.4 months [17] and 10.5 months [10]. A 67-month interval appears to be the longest interval reported [18]. Extramedullary AML appears more common in case of hyperleucocytosis, core binding factor (CBF) leukemia’s, AML with a monocytic component, CD56 leukemia cell positivity and in long-term survivors (CBF leukemia’s and patients allografted) in relationship with leukemia cell persistence in certain sites because of natural barriers or a specific microenvironment. It was also stated that the incidence of granulocytic sarcoma is twice as high in patients with chronic myeloid leukemia (CML) than in those with AML [10]. In the largest series of myeloid sarcomas, chromosomal aberrations were detected in 54% of cases [2]. Monosomy 7 (10.8%), trisomy 8 (10.4%) and MLL-spitting (8.5%) were the commonest abnormalities, whereas t (8; 21) was rare (2.2%). However, review of the literature highlights that such translocation has been detected in tumors occurring more often in childhood and/or at the orbital level [19,20].
The differential diagnosis involves mainly inflammatory lesions and a variety of malignant tumors, of which the most common incorrect diagnoses include malignant lymphoma, small cell carcinoma, sarcoma, and undifferentiated tumor. Leukemia cells generally do not disturb the natural architecture of the tissue, while there is tissue destruction and necrosis in lymphoma [21]. In granulocytic sarcoma, the borders of the nuclear outline of cells are irregular, the cytoplasm is eosinophilic, and eosinophilic myelocytes are predominant which could also be observed in T-cell lymphomas [22]. A number of antibodies react with myeloid cells and can be used for immunochemical analyses [23]. Most of the misdiagnoses were made on patients whose tumor preceded the overt leukemia [10]. Immunophenotyping appears then crucial for making the diagnosis. CD43 is expressed in most myeloid sarcomas. Granulocytic sarcomas also expressed CD13, CD33, CD117 and myeloperoxydase. Monocytic sarcomas are myeloperoxydase negative but express CD68, CD163 and lysozyme.
In the review of 89 cases from the literature (Table 1), the initial site of involvement was the uterine cervix in 37 cases (42%), the ovary in 22 cases (25%), and the vagina in 8 cases (9%). The other anatomic sites involved were uterus corpus (11 cases), labia majora (1 case), clitoris (1 case), and adnexal (1 case). In the other cases, the site of origin was not always precisely determined between uterus and ovary (3 cases), vulva and vagina (2 cases), uterine cervix and ovary (1 case), uterus and adnexal (1 case), and ovary and omentum (1 case). Median age at diagnosis was 42.5 years (range: 1 week – 76 years). Twenty cases of myeloid sarcoma of the gynecologic tract (22%) reported a prior history of myeloid neoplasm. In 33 cases (37%), there was a simultaneous bone marrow involvement by AML. In 43 cases (48%), the female genital tract was the only site of disease at the time of presentation without any known history of hematologic malignancy. When the uterus is involved, the disease is usually limited to the cervix. Involvement of uterine body is uncommon. As it was the case in our patient, these tumors can be locally invasive and can cause ureteric obstruction. Granucytic sarcoma of the genital tract appeared more frequent in women than in men [8]. There is no clear explanation for this male/female discrepancy. However, several publications have reported concomittant granulocytic sarcoma of the female gynecologic tract and granulocytic sarcoma in breasts [24- 27]. It has then been postulated that some AML may have steroid hormonal receptors [27], which was not confirmed further. The type of AML was not always indicated. However, several cases reported CBF leukaemia’s [16,28-36]. The mechanism of the occurrence of granulocytic sarcoma may be related to the deregulation of CBF transcription factors involved in cell recognition and adhesion. This type of leukemia has also been reported to form solid tumors in the central nervous system [37]. Despite an overall favourable prognosis of CBF leukemia subtype when treated with intensive chemotherapy including high-dose cytarabine, it remains uncertain what constitutes the best treatment in this specific situation. High-dose chemotherapy and stem cell transplantation may benefit for these patients [31]. Similarly, monoblastic sarcomas with 11q23/MLL rearrangements have often been observed [38]. This supports a therapy-related origin of the myeloid sarcoma. Leukaemia’s with MLL gene rearrangement have been reported after chemotherapies that include topoisomerase II inhibitors [39]. In our case report, MLL gene rearrangement was identified at the time of relapse, while it was not present at the time of initial diagnosis.
Reference
Site
AML
Previous/concurrent
Age
Treatment
[42]
Uterine cervix
No/Yes
26
None
[43]
Uterine cervix
No/NS
39
Rad
[44]
Uterine cervix, ovary
Yes/Yes
50
TAH-BSO, Chemo
[26]
Vagina
Yes/No
13
Chemo
[45]
Uterine cervix
No/No
44
TAH-BSO
Uterine cervix
No/No
75
Rad
Ovary
No/NS
37
SO, Chemo
[46]
Ovary
No/No
28
SO
[47]
Ovary
No/No
44
SO
[24]
Vulva, vagina
No/Yes
24
Steroids
[48]
Uterine cervix
No/No
65
Rad
[25]
Vulva, vagina
No/Yes
45
Chemo
[49]
Uterine cervix
No/Yes
58
None
[50]
Ovary
Yes/Yes
18
Chemo
[51]
Uterine cervix
No/Yes
36
TAH-BSO
[52]
Ovaries
No/Yes
1 week
SO, Chemo
[53]
Uterine cervix
No/No
39
None
[27]
Vagina
Yes/Yes
34
Chemo, Rad
[54]
Uterine cervix
No/Yes
59
None
Uterine cervix
No/No
59
Rad
[55]
Uterine cervix
No/No
40
Chemo, Rad
Uterine cervix
No/No
48
TAH-BSO, Chemo
[56]
Uterine cervix
No/No
34
Chemo
[57]
Uterine cervix
Yes/No
33
None
[58]
Vagina
Yes/No
53
Chemo, Rad
[40]
Uterine cervix
No/No
43
Chemo
Uterus, adnexal
No/No
17
Rad
[59]
Adnexal tumor
Yesb/Yes
36
Steroids
[60]
Uterine cervix
No/No
32
Rad
[61]
Uterine cervix
No/No
66
Chemo
[62]
Ovary
No/Yes
46
None
[63]
Ovary
No/No
49
NS
[17]
Uterine cervix
No/Yes
51
Chemo
[64]
Ovary
No/No
31
Chemo
[65]
Uterine cervix
No/No
48
Chemo
[33]
Ovary
No/Yes
17
Chemo, oophorectomy
Ovary, omentum
No/Yes
46
Exploratory laparotomy, Chemo
[66]
Uterine cervix
No/No
20
Chemo, hysterectomy, AutoSCT, Rad
[67]
Uterine cervix
Yes/Yes
35
Chemo
[68]
Uterine cervix
No/No
33
Death before treatment
[11]
Ovaries
No/No
30
Bilateral oophorectomy
Ovary
No/No
31
Oophorectomy, omentectomy, Chemo
Ovary
No/No
31
Oophorectomy
Ovary
No/No
43
Oophorectomy, Chemo
Vagina
No/No
73
Rad
Vagina
No/Yes
66
Chemo
Vagina
Yes/Yes
76
Chemo
Uterine cervix
No/No
NS
TAH
Ovary
Yes/No
25
Oophorectomy, Chemo
Ovaries
No/Yes
13
Oophorectomy, Chemo
Ovary
No/Yes
35
Oophorectomy
[69]
Uterus
No/Yes
73
NS
[70]
Uterine cervix
No/No
41
Chemo
[71]
Uterine cervix
No/No
49
Rad, Chemo
[72]
Uterine cervix
Yes/No
67
Chemo
[73]
Ovary
No/No
12
NS
[74]
Ovary
No/No
26
Chemo
[75]
Uterine cervix
No/No
48
Chemo, hysterectomy, oophorectomy, AutoSCT
Vagina
No/No
48
Chemo, hystectomy, surgery of proximal vagina, AutoSCT
[32]
Uterine cervix
Yes/No
30
Chemo
[34]
Ovary
No/Yes
35
Chemo
[76]
Uterine cervix
Yes/No
30
Chemo
[16]
Uterine cervix
No/No
33
Chemo
[77]
Ovary
No/Yes
42
Chemo
[78]
Uterus
Yes/Yes
50
Rad
[35] a
Uterine cervix
No/No
37
Chemo
Uterine cervix
No/No
34
Chemo
Uterus
No/No
52
NS
Uterus, ovary
No/No
35
Chemo, AlloSCT
Ovary
No/No
25
Chemo
Ovary
No/Yes
44
NS
Clitoris
Yes/No
60
Chemo
Uterus, ovaries
No/No
59
Chemo
Uterus
Yes/Yes
46
Chemo
Uterine cervix
No/No
43
Chemo
Uterus, ovary
No/No
17
Rad
[38]
Uterus
No/No
49
Chemo, AlloSCT
[79]
Uterus
No/Yes
NS
Chemo
[80]
Uterus
Yes/No
49
NS
[36]
Uterus
No/Yes
50
Chemo
[81]
Labia majora, vulva
No/Yes
73
Chemo
[82]
Uterus
No/Yes
NS
Chemo, TAH-BSO
[83]
Uterine cervix
No/Yes
61
Chemo
[84]
Uterus
No/Yes
62
Chemo
[85]
Vagina
Noc/No
52
NS
[86]
Uterine cervix
No/No
30
Chemo, Rad
[30]
Uterus
Yes/No
55
Hysterectomy, Rad
[87]
Uterine cervix
Yes/No
23
Chemo
Present Case
Uterine cervix
Yes/Yes
58
Chemo, Rad, AlloSCT
Abbreviations: AlloSCT: Allogeneic Stem Cell Transplantation; AutoSCT: Autologous Stem Cell Transplantation; BSO: Bilateral Salpingo-oophorectomy; Chemo: Chemotherapy; NS: Not Stated; Rad: Radiation; SO: Salpingo-oophorectomy; TAH: Total Abdominal Hysterectomy.
aSix patients underwent total abdominal hysterectomy (2 with bilateral and 2 with unilateral salpingo-oophorectomy), and 2 patients had oophorectomy; bAntecedents of chronic myeloid leukemia, granulocytic sarcoma of the female genital tract developed as manifestation of blastic transformation; c No antecedent of AML, but granulocytic sarcoma of the uterine cervix 11 years earlier.
Table 1: Review of the literature regarding myeloid sarcoma involving the female genital tract.
Our observation suggests that despite evidence of localized disease, myeloid sarcoma is indicative of widespread systemic disease and should be treated accordingly. Treatment should be centred on the underlying leukemia. Treatment of uterine myeloid sarcoma has been described with combinations of surgery, radiation and chemotherapy. In the current case, surgery was avoided because of the wide tumor involvement. It has been shown that patients with no discernible hematologic abnormality at diagnosis who received no chemotherapy developed AML in more than 80% of cases within 11 months [10,29]. Reversely, it was demonstrated that almost half of the patients who received chemotherapy remained in a non-leukemic stage for a long period of time [29,40]. Therefore, cases presenting as isolated myeloid sarcoma should receive similar therapy as for AML with the same cytogenetic abnormality. Granulocytic sarcoma responds well to radiotherapy, but it is unclear whether radiotherapy improves survival [41]. The role of local therapy such as radiation remains unknown. Irradiation therapy did not seem to improve the disease free interval and the prognosis, but could be discussed in case of residual sites of malignant activity after chemotherapy. Granulocytic sarcoma of the female genital tract appears to have a prognosis similar to that of granulocytic sarcomas at other body sites. Surgical resection of the bulk of the tumor has only a diagnostic interest, but because of obvious residual disease, patients should receive adjuvant chemotherapy. Extramedullary disease is often considered as an adverse prognostic factor in AML [2]. Although the occurrence of a granulocytic sarcoma in a patient with AML does not seem to alter the prognosis [10] most if not all long survivors received stem cell transplantation. Myeloid sarcoma would therefore represent an indication of treatment by allogeneic hematopoietic stem cell transplantation.
References
- Jaffe ES, Harris NL, Stein H, Vardimann JW, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon. France: IARC Press, 2001.
- Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007; 21: 340-350.
- Burns A. Observations of surgical anatomy. Head and neck. Royce, Edinburgh, 1811.
- King A. A case of chloroma. Monthly J Med. 1853; 17:97.
- Dock G. Chloroma and its relation to leukemia. Am J Med Sci. 1983; 106: 152.
- Burgess AM. Chloroma. J Med Res. 1912; 27:133.
- Rappaport H. Tumors of the hematopoietic system: Atlas of tumor pathology. Section III, fascicule 8, Armed Forces Institute of Pathology, Washington DC. 1966; 241-243.
- Liu PI, Ishimaru T, McGregor DH, Okada H, Steer A. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949-1969. Cancer. 1973; 31: 948-955.
- Muss HB, Moloney WC. Chloroma and other myeloblastic tumors. Blood. 1973; 42:721-728.
- Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsed cases. Cancer. 1981; 48: 1426-1437.
- Oliva E, Ferry JA, Young RH, Prat J, Srigley JR, Scully RE. Granulocytic sarcoma of the female genital tract: a clinicopathologic study of 11 cases. Am J Surg Pathol. 1997; 21:1156-1165.
- Thomas X, Elhamri M, Raffoux E, Renneville A, Pautas C, de Botton S, et al. Comparison of high-dose cytarabine and timed-sequential chemotherapy as consolidation for younger adults with AML in first remission: the ALFA-9802 study. Blood. 2011; 118: 1754-1762.
- Belhabri A, Thomas X, Wattel E, Chelghoum Y, Anglaret B, Vekhoff A, et al. All trans-retinoic acid in combination with intermediate-dose cytarabine and idarubicin in patients with relapsed or refractory non promyelocytic acute myeloid leukemia: A phase II randomized trial. Hematol J. 2002; 3: 49-55.
- Scheidawind D, Federmann B, Faul C, Vogel W, Kanz L, Bethge WA. Allogeneic hematopoietic cell transplantation with reduced-intensity conditioning following FLAMSA for primary refractory or relapsed acute myeloid leukemia. Ann Hematol 2013; 92: 1389-1395.
- Hutchison RE, Kurec AS, Davey FR. Granulocytic sarcoma. Clin Lab Med. 1990; 10: 889-901.
- Pathak B, Bruchim I, Brisson ML, Hammouda W, Bloom C, Gotlieb WH. Granulocytic sarcoma presenting as tumors of the cervix. Gynecol Oncol. 2005; 98: 493-497.
- Friedman HD, Adelson MD, Elder RC, Lemke SM. Granulocytic sarcoma of the uterine cervix – Literature review of granulocytic sarcoma of the female genital tract. Gynecol Oncol. 1992; 46: 128-137.
- Beck TM, Day JC, Smith CE, Eddy HE. Granulocytic sarcoma treated as an acute leukemia: Report of a case. Cancer. 1984; 53: 1764-1766.
- Schwyzer R, Sherman GG, Cohn RJ, Poole JE, Willem P. Granulocytic sarcoma in children with acute myeloblastic leukaemia and t(8;21). Med Pediatr Oncol. 1998; 31: 144-149.
- Rubnitz JE, Raimondi SC, Halbert AR, Tong X, Srivastava DK, Razzouk BI, et al. Characteristics and outcome of t(8;21)-positive childhood acute myeloid leukaemia: a single institution’s experience. Leukemia. 2002; 16: 2072-2077.
- Chang CC, Eshoa C, Kampalath B, et al. Immunophenotypic profile of myeloid cells in granulocytic sarcoma by immunohistochemistry. Correlation with blast differentiation in bone marrow. Am J Clin Pathol. 2000; 114: 807-811.
- Menasce LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathol. 1999; 34: 391-398.
- Byrd JC, Weiss RB. Recurrent granulocytic sarcoma: an unusual variation of acute myelogenous leukemia associated with 8; 21 chromosomal translocation and blast expression of the neural cell adhesion molecule. Cancer. 1994; 73: 2107-2112.
- Gardais J, marie M, Bertrand G. Chlorome à localisations génitales multiples. Sem Hôp Paris 1975; 51:609-616.
- Laricchia R, Wierdis T, Loiudice L, Trisolini A, Riezzo A. Neoformazione vulvare (mieloblastoma) coma prima manifestazione di una leucemia acuta mieloblastica. Minerva Ginecol 1977 ; 29:957-961.
- Gralnick HR, Dittmar K. Development of myeloblastoma with massive breast and ovarian involvement during remission in acute leukemia. Cancer. 1969; 24: 746-749.
- Socinski MA, Ershler WB, Belinson JL. Coexistent breast and vaginal granulocytic sarcoma. Gynecol Oncol. 1983; 16: 299-304.
- Wodzinski MA, Collin R, Winfield DA, Dalton A, Lawrence ACK. Epidural granulocytic sarcoma in acute myeloid leukemia with 8;21 translocation. Cancer. 1988; 62: 1299-1300.
- Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma. Report of two cases and a review of 72 cases in the literature. Cancer. 2002; 94: 1739-1746.
- Zaenker S, Schweyer S, Hasenkamp J, Truemper L, Wulf G. Granulocytic sarcoma by AML M4Eo (inv16) after allogeneic stem cell transplantation without bone marrow involvement. Case Rep Hematol. 2011; 2011: 692982.
- Zhang XH, Zhang R, Li Y. Granulocytic sarcoma of abdomen in acute myeloid leukemia patient with inv(16) and t(6;17) abnormal chromosome: case report and review of literature. Leuk Res 2010; 34:958-961.
- Maeng H, Cheong JW, Lee ST, Yang WI, Hahn JS, Ko YW, et al. Isolated extramedullary relapse of acute myelogenous leukemia as a uterine granulocytic sarcoma in an allogeneic hematopoietic stem cell transplantation recipient. Yonsei Med. J 2004; 45: 330-333.
- Drinkard LC, Waggoner S, Stein RN, Byrne RA, Larson RA. Acute myelomonocytic leukemia with abnormal eosinophils presenting as an ovarian mass: a report of two cases and a review of the literature. Gynecol Oncol. 1995; 56: 307-311.
- Yavuz S, Paydas S, Disel U, Erdogan S. Ovarian granulocytic sarcoma. Leuk Lymphoma 2004; 45: 183-185.
- Garcia MG, Deavers MT, Knoblock RJ, Chen W, Tsimberidou AM, Manning JT, et al. Myeloid sarcoma involving the gynecologic tract. A report of 11 cases and review of the literature. Am J Clin Pathol. 2006; 125: 783-790.
- Mallo M, Espinet B, Salido M, Ferrer A, Pedro C, Besses C, et al. Gain of multiple copies of the CBFB gene: a new genetic aberration in a case of granulocytic sarcoma. Cancer Genet Cytogenet. 2007; 179: 62-65.
- Glass JP, Van Tassel P, Keating MJ, et al. Central nervous system complications of a newly recognized subtype of leukemia: acute myelomonocytic leukemia with a pericentric inversion of chromosome 16. Neurology. 1987; 37: 639-644.
- Pullarkat V, Veliz L, Chang K, Mohrbacher A, Teotico AL, Forman SJ, et al. Therapy-related, mixed-lineage leukaemia translocation-positive, monoblastic myeloid sarcoma of the uterus. J Clin Pathol. 2007; 60: 562-564.
- Leone G, Voso MT, Sica S, Morosetti R, Pagano L. Therapy related leukemias: susceptibility, prevention and treatment. Leuk Lymphoma. 2001; 41: 255-276.
- Meis JM, Butler JJ, Osborne BM, Manning JT. Granulocytic sarcoma in nonleukemic patients. Cancer. 1986; 58: 2697-2709.
- Chak LY, Sapozink MD, Cox RS. Extramedullary lesions in non-lymphocytic leukemia: results of radiation therapy. Int J radiat Oncol Biol Phys. 1983; 9: 1173-1176.
- Schlagenhauffer F. Ein fall von chloro-leukämie mit grünem uterus. Arch Gynaekol 1912; 95:1-12.
- Bérard L, Martin JF, Ponthus P. Etude d’un myélo-sarcome (hémocystoblastosarcome myéloïde) du col utérin et de ses généralisations squelettiques. Bull Assoc Fr Etude Cancer. 1936; 25:611-620.
- Hartford CE. Bleeding form the uterus caused by chloroma. Obstet Gynecol. 1968; 31: 166-172.
- Chorlton I, Karnei RF, King FM, Norris HJ. Primary malignant reticuloendothelial disease involving the vagina, cervix, and corpusuteri. Obstet Gynecol. 1974; 44: 735-748.
- Schafer RJ, Hill K, Reitz H. Myelosarcoma of the ovary preceeding leukemia [in German]. Zentralbl Allg Pathol. 1974; 118: 467-472.
- Kahn K. Diagnostisch irreführender ovarialtumor. Wein Klin Wochenschr. 1975; 87:55-56.
- Seo IS, Hull MT, Pak HY. Granulocytic sarcoma of the cervix as a primary manifestation: case without overt leukemic features for 26 months. Cancer. 1977; 40: 3030-3037.
- Kapadia SB, Krause JR, Kanbour AI, Hartsock RJ. Granulocytic sarcoma of the uterus. Cancer. 1978; 41: 687-691.
- Ballon SC, Donaldson RC, Berman ML, Swanson GA, Byron RL. Myeloblastoma (granulocytic sarcoma) of the ovary. Arch Pathol Lab Med. 1978; 102: 474-476.
- Park CI, Kim TS, Lee YB. Granulocytic sarcoma of the uterine cervix preceding myelogenous leukemia. Yonsei Med J. 1980; 21: 36-42.
- Morgan ER, Labotka RJ, Gonzalez-Crussi F, Wiederhold M, Sherman JO. Ovarian granulocytic sarcoma as the primary manifestation of acute infantile myelomonocytic leukemia. Cancer. 1981; 48: 1819-1824.
- Spahr J, Behm FG, Schneider V. Preleukemic granulocytic sarcoma of cervix and vagina: initial manifestation by cytology. Acta Cytol. 1982; 26: 55-60.
- Abeler V, Kjorstad KE, Langholm R, Marton PF. Granulocytic sarcoma (chloroma) of the uterine cervix: report of two cases. Int J Gynecol Pathol. 1983; 2: 88-92.
- Harris NL, Scully RE. Malignant lymphoma and granulocytic sarcoma of the uterus and vagina: a clinicopathologic analysis of 27 cases. Cancer. 1984; 53: 2530-2545.
- Kao KL, Yang YS, Lee CJ, Shieh HT, Lee EF, Chen YC. Granulocytic sarcoma of the cervix uteri: a case report. Taiwan Yi Xue Hui Za Zhi. 1984; 83: 618-625.
- Urbano-Ispizua A, Sierra J, Ribera JM, Lailla J, Granena A, Rozman C. Sarcoma granulocitico en cervix uterino como forma de recaida de una leucema aguda mieloblastica (L.A.M.). Sangre. 1985 ; 30 : 936-937.
- Steinbock GS, Morrisseau PM, Vinson RK. Acute obstructive renal failure secondary to granulocytic sarcoma (chloroma). Urology. 1986; 27: 268-270.
- Sagar TG, Maitreyan V, Majhi U, Shanta V. Ovarian granulocytic sarcoma in chronic granulocytic leukaemia with myeloblastic crisis. J Assoc Physicians India. 1989; 37: 542-543.
- Banik S, Grech AB, Eyden BP. Granulocytic sarcoma of the cervix: an immunohistochemical, histochemical, and ultrastructural study. J Clin Pathol. 1989; 42: 483-488.
- Desablens B, Lesoin A, Bourret A, Peltier F, Sevestre H, Closset P. Gynecologic and breast granulocytic sarcoma. Review of the literature. Apropos of a case in the cervix uteri. J Gynecol Obstet Biol Reprod. 1990; 19: 857-861.
- Magliocco AM, Demetrick DJ, Jones AR, Kossakowska AE. Granulocytic sarcoma of the ovary. Arch Pathol Lab Med. 1991; 115: 830-834.
- Pressler H, Horny HP, Wolf A, Kaiserling E. Isolated granulocytic sarcoma of the ovary: histologic, electron microscopic, and immunohistochemical findings. Int J Gynecol Pathol. 1992; 11: 68-74.
- Aguiar RC, Pozzi DH, Chamone DA. Granulocytic sarcoma of the ovary in a nonleukemic patient. Haematologica. 1993; 78: 53-55.
- Reynaud P, Le Bouëdec G, Déchelotte P, Dauplat J, Chassagne J, Fonck Y. Rare tumors of the cervix: three case reports: rhabdomyosarcoma, granulocytic sarcoma and lymphoma [in French]. J Gynecol Obstrt Biol Reprod (Paris). 1995; 24: 30-34.
- Huter O, Brezinka C, Nachbaur D, Schwaighofer H, Lang A, Niederwieser D. Successful treatment of primary extramedullary leukemia (EML) of the uterus with radical surgery, chemotherapy, autologous bone marrow transplantation (BMT) and prophylactic local irradiation. Bone Marrow Transplant. 1996; 18: 663-664.
- Wong KF, Yu PH, Chu YC. Granulocytic sarcoma of the uterus complicating myelodysplastic syndrome. Am J Hematol. 1996; 51: 244-245.
- Kamble R, Kochupillai V, Sharma A, Kumar L, Thulkar S, Sharma MC, et al. Granulocytic sarcoma of uterine cervix as presentation of acute myeloid leukemia: a case report and review of literature. J Obstetr Gynaecol Res. 1997; 23: 261-266.
- Glaser C, Michelon B, Peltier JY, Duboucher C, Bernheim A, Périé G. Pre-leukemic granulocytic sarcoma of the uterus [in French]. Report of a case. Ann Pathol. 1998; 18: 187-191.
- Lu X, Xiao L. Granulocytic sarcoma (chloroma) of the uterine cervix : a case report. Chinese Med J. 1999; 112: 858-860.
- Mwanda WO, Rajab JA. Granulocytic sarcoma: report of three cases. East Afr Med J. 1999; 76: 594-596.
- Delaflor-Weiss E, Zauber NP, Kintiroglou M, Bermon EL, Malcynski D. Acute myelogenous leukemia relapsing as granulocytic sarcoma of the cervix. Acta Cytol. 1999; 43: 1124-1130.
- Jung SE, Chun KA, Park SH, Lee EJ. MR findings in ovarian granucytic sarcoma. Br J Radiol. 1999; 72: 301-303.
- Sreejith G, Gangadharan VP, Elizabath KA, Preetha S, Chithrathara K. Primary granulocytic sarcoma of the ovary. Am J Clin Oncol. 2000; 23: 239-240.
- Hernadez JA, Navarro JT, Rozman M, Ribera JM, Rovira M, Bosch MA, et al. Primary myeloid sarcoma of the gynecologic tract: a report of two cases progressing to acute myeloid leukemia. Leuk Lymphoma. 2002; 43: 2151-2153.
- Lee JW, Kim YT, Min YH, Kim JW, Kim SH, Park KH, et al. Granulocytic sarcoma of the uterine cervix. J Gynecol Cancer. 2004; 14: 553-557.
- Tripathi R, Sharma B, Chaturvedi KU, Khurana N, Mala YM. Granulocytic sarcoma of the female genital tract: report of a case with an unusual presentation. Gynecol Obstr Invest. 2005; 59: 189-191.
- Pitz MW, Maslyak O, Morales C, Seftel MD. Myeloid sarcoma of the uterus presenting as vaginal bleeding. Intern Med J. 2006; 36: 669-671.
- Usmani SZ, Shahid Z, Saleh H, Nasser KA. Myeloid sarcoma presenting with acute renal and bilateral ureteral obstruction: a case report and review of the literature. Am J Med Sci. 2007; 334: 136-138.
- Lambein K, Janssens A, Weyers S, Praet M, De Paepe P. Presence of myeloid precursor cells in the endometrium of an AML patient: a diagnostic challenge! Virchows Arch. 2007; 451: 1097-1098.
- Ersahin C, Omeroglu G, Potkul RK, Salhadar A. Myeloid sarcoma of the vulva as the presenting symptom in a patient with acute myeloid leukemia. Gynecol Oncol. 2007; 106: 259-261.
- Kilic G, Boruban MC, Bueco-Ramos C, Konoplev SN. Granulocytic sarcoma involving the uterus and right fallopian tube with negative endometrial biopsy. Eur J Gynaecol Oncol. 2007; 28: 270-272.
- Gregor M, Tomsova M, Siroky O. Granulocytic sarcoma of the uterus. Int J Gynaecol Obstet. 2008; 103: 73-74.
- Guile MW, Vuica-Ross M, Sewell C. Granulocytic sarcoma of the endometrium. Female Patient 2008; 33:45-46.
- Chiang YC, Chen CH. Cervical granulocytic sarcoma: report of one case and review of the literature. Eur J Gynaecol Oncol. 2010; 31: 697-700.
- Kim SC, Natarajan-Ame S, Lioure B, Chenard MP, Duclos B, Herbrecht R, et al. Successful treatment of a granulocytic sarcoma of the uterine cervix in complete remission at six-year follow-up. J Oncol. 2010; 2010: 812424.
- Ucar M, Guryildirim M. Granulocytic sarcoma of the uterus: a rare presentation of extramedullary relapse of AML and importance of MRI. Case Rep Radiol. 2014; 2014: 501342.