
Case Report
Ann Hematol Oncol. 2015; 2(8): 1060.
Complete Remission with α-Interferon in a Poor Risk Patient with Blastic Plasmocytoid Dendritic Cell Neoplasm: A Case Report and Review of Literature
Mazziotta F¹*, Caracciolo F¹, Orciuolo E¹, Buda G¹, Benedetti E¹, Bonadio AG², Iovino L¹, Carulli G¹, Ciancia EM³, Azzarà A¹, Galimberti S¹ and Petrini M¹
¹Department of Oncology, Transplant and Advances in Medicine–Section of Haematology, University of Pisa, Italy
²First Division of Pathology, University of Pisa, Italy
³Second Division of Pathology, University of Pisa, Italy
*Corresponding author: Francesco Mazziotta, Department of Oncology, Transplant and Advances in Medicine–Section of Haematology, University of Pisa, Italy
Received: September 17, 2015; Accepted: November 15, 2015; Published: November 17, 2015
Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and clinically aggressive haematologic malignancy caused by neoplastic transformation and clonal proliferation of plasmacytoid dendritic cell precursors. Current treatment approaches are not effective and the outcome remains poor in the majority of patients even after chemotherapy and bone marrow transplantation. In this report we consider the use of interferon alpha for old unfit patients not candidate for intensive strategies.
Keywords: Blastic plasmacytoid dendritic cell neoplasm; Interferon alpha; NK
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN), due to its biological and clinical features, could be classified as an intermediate disease between acute myeloid leukemia (WHO 2008) [1] and non Hodgkin lymphomas (WHO-EORTC 2005) [2].
In the first BPDCN case published in 1994 [3], authors reported the lack of a lineage specific antigen and the co-expression of CD4 and CD56 suggesting a natural killer (NK) cell-lineage origin. This entity was firstly defined as blastic NK cell lymphoma (BNKL) [4]. Later, the discovery of CD123 (interleukin-3 receptor) and BDCA2 (blood dendritic cell antigen 2) expression allowed to identify these cells as plasmocytoid dendritic cells. Blastic cells appear to secrete α-Interferon (INF) [5-7] and thus are immunophenotipically and functionally identified as immature dendritic cells. For the above mentioned reasons, the tumor was renamed blastic plasmacytoid dendritic cell neoplasm [1].
Plasmocytoid dendritic cells (pDCs) develop in bone marrow, reside primarily in the lymphoid organs (nodes and thymus) in steady state and can be mobilized, in response to some stimula including G-CSF and FLT-3, into peripheral blood, where they are poorly represented (< 1% of the mononuclear cells).
In lymph nodes pDCs are detectable in T-cell rich areas [8,9] as round or oval cells with an eccentric nucleus and a well developed endoplasmic reticules (ER).
Fred Siegal and Yong-Jun Liu [10] demonstrated that these secretory cells produced large amounts of IFN and identified them with the IFN-producing cells previously described [11-13].
As components of the innate immune system, pDCs detect viral acid nucleic through toll-like receptor 7 (TLR7) and toll-like receptor 9 (TLR-9), inducing the IFN secretion. This protein is responsible for the resistance to viral infections and promotes NK, DC, T and B cell functions [14].
IFN signaling promotes the survival of ex vivo pDCs [15,16], but in vivo studies [17] have demonstrated that IFN mediates pDCs death through caspase activation, creating a proapoptotic loop.
Limited data are available to guide the management of BPDCN. Response to conventional chemotherapy is commonly poor and survival correspondingly short (mean survival of 12-14 months) [18].
Several Authors suggest the effectiveness of allogeneic hematopoietic stem cell transplantation (AHSCT) [19-21].
Case Presentation
A 74-year-old man was admitted on our chemotherapy ward because of BPDCN with initial skin involvement.
At diagnosis, in November 2013, the patient showed 2-3 cm purplish eruptive nodules, neither painful nor itchy, preferentially located on his trunk and legs (Figure 1).
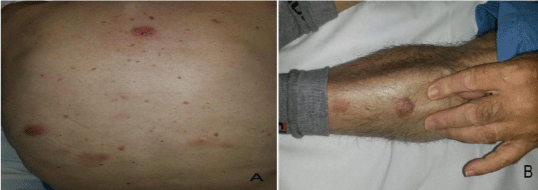
Figure 1: Multiple nodules on the (A) trunk and (B) leg.
A biopsy specimen taken from thigh skin lesion revealed a dermoepidermal infiltrate of small sized cells (Figure 2) reactive to CD4 and CD56. Immunostaining for CD8, CD30, CD20, CD79a, CD3, CD5, CD10, CD23, Bcl-6, TdT, myeloperoxidase, D1 cyclin, CD34, CD117 and CD138 was negative. Mib-1 proliferation index was 70%.
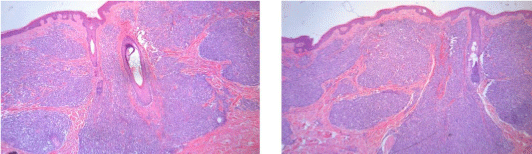
Figure 2: Skin biopsy: haematoxylin and eosin staining shows a dermoepidermal
infiltrate of small sized cells.
All these features were consistent with the diagnosis of BPDCN.
The patient underwent staging with complete blood counts, blood chemistry, total body computer tomography scan and bone marrow biopsy. Blood counts and serum chemistry were within normal range and CT imaging was negative for lymph nodes involvement or other noteworthy. Bone marrow appeared infiltrated (15%) by mediumsized blast-like cells with basophilic cytoplasm and irregular nuclei. Flow cytometry identified a low percentage (3%) of immature elements CD34+/CD38+/CD56+ and molecular characterization showed a clonal T cell receptor delta (TCR) gene rearrangement. The patient underwent active surveillance until April 2014, when he developed clinical progression with enhanced skin involvement, occurrence of enlarged neck lymph nodes, tonsil involvement and increased bone marrow infiltration (blasts CD4+/CD56+ 35%)(Figure 3).
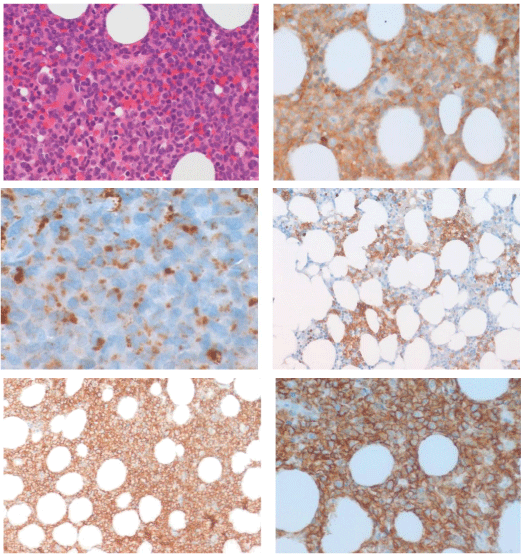
Figure 3: (A) Haematoxylin and eosin staining and immunohistochemical
staining for (B) CD4+ 200x (C) CD68+ (D,E) CD56+ 200x (F) CD56+ 400x.
Age and comorbidities (dysmetabolism and previous nephrectomy for a kidney malignancy) did not candidate the patient for intensive chemotherapy followed eventually by allotransplant.
The patient underwent therapy with subcutaneous administration of α-INF 3.0 MUI three times per week for two months and oral administration of dexametasone 20 mg on day 1-4 of each month. He was monitored monthly by measuring the diameter of the skin lesions and repeated bone marrow blast count with molecular analysis at +3 and +6 months (Figure 4).
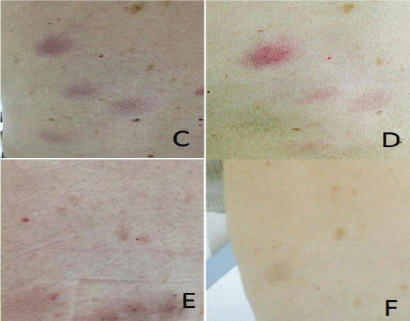
Figure 4: Clinical response. (C) Diagnosis, (D) +1 months, (E) +3 months, (F)
+8 months from the beginning of therapy.
Skin nodules and lymph nodes progressively reduced within 4 weeks from therapy beginning. After a year and a half from the diagnosis our patient is alive, without major side effects and in complete response with respect to bone marrow and lymph node diseases. Bone marrow analysis shows complete morphologic, immunophenotipic and molecular response (TCR delta rearrangement negativity). Skin lesions are now present in the form of small dyschromic alterations.
Discussion
BPDCN is a rare, aggressive and incurable malignancy, derived from plasmacytoid dendritic cells precursors and characterized by co-expression of CD4 and CD56. Its incidence in the population is unknown due to the lack of defining criteria. Literature reports very low incidence (0.44% of all hematologic malignancies) and rare leukemic presentation: <1% of cases of acute leukemia. BPDCN affects males prevalently, with a male/female ratio of 3.5/1, and generally occurs in older adults, with a median age of 60 years at diagnosis [22].
In the majority of cases patients present solitary or multiple nonpruritic skin lesions and lymph nodes enlargements, with subsequent or rarely simultaneous leukemic spread.
In the last two decades many efforts were made in order to understand the underlying biology of BPCDN, indentifying DCs as the normal counterpart of CD4+CD56+ malignant cells [23,24]. Still little is known about cancer initiation, promotion and leukemic progression making a pathogenesis-based therapy not available. Moreover the absence of prospective data makes treatment of this neoplasm a great challenge to physicians.
In agreement with literature, intensive strategies [19-21] possibly followed by allogeneic transplantation should be considered the best choice in order to produce deep and prolonged responses.
Responses obtained after front-line therapy are variable ranging from 40-50% for CHOP-like chemotherapy [25] to 80-90 % for AML/ ALL like regimens [26]. However, independently of the frontline chemotherapy, responses are short-lived [18-20,27] and clinical course is usually characterized by an initial response followed by relapse.
In this work we present a case of BPDCN with typical skin lesions at presentation, followed by bone marrow involvement and leukemic progression. Due to patient’s age and comorbidities he didn’t undergo multiagent chemotherapy and allogeneic HSCT.
In the absence of a standard of care, awareness of the aggressive behavior of BPDCN and of the need for ongoing treatment, we have considered the use of α-INF in this disease.
This drug is easy to administer, doesn’t require hospitalization and it is charged by infrequent minor side effects (flu-like symptoms, hematological toxicity, elevated transaminases, nausea) usually adequately handled by dose adjustment.
Alpha IFN administration in BPDCN appears to have a biological rationale. Some in vivo [17] studies have shown that when skin-DCs are exposed to high concentrations of α-IFN, they undergo apoptosis. On the basis of these results and considering the effectiveness of IFN in several lymphoid and myeloid diseases, our patient underwent α-IFN therapy. Considering mean survival reported in the literature after intensive strategies, this drug in our patient demonstrated to be no less effective.
We suggest that α-IFN could be an option to consider for old unfit patients that cannot be exposed to conventional multidrug chemotherapy.
References
- Faccheti F, Jones DM, Petrella T. Blastic plasmacytoid dendritic cell neoplasm. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2008. pp. 145–147.
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005; 105: 3768-3785.
- Adachi M, Maeda K, Takekawa M, Hinoda Y, Imai K, Sugiyama S, et al. High expression of CD56 (N-CAM) in a patient with cutaneous CD4-positive lymphoma. Am J Hematol. 1994; 47: 278-282.
- Chan JKC, Jaffe ES, Ralfkiser E. Blastic NK-cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. WHO classification. Tumors of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001. p 214–215.
- Pilichowska ME, Fleming MD, Pinkus JL, Pinkus GS. CD4+/CD56+ hematodermic neoplasm ("blastic natural killer cell lymphoma"): neoplastic cells express the immature dendritic cell marker BDCA-2 and produce interferon. Am J Clin Pathol. 2007; 128: 445-453.
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999; 5: 919-923.
- Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000; 192: 219-226.
- LENNERT K, REMMELE W. [Karyometric research on lymph node cells in man. I. Germinoblasts, lymphoblasts & lymphocytes]. Acta Haematol. 1958; 19: 99-113.
- Feller AC, Lennert K, Stein H, Bruhn HD, Wuthe HH. Immunohistology and aetiology of histiocytic necrotizing lymphadenitis. Report of three instructive cases. Histopathology. 1983; 7: 825-839.
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999; 284: 1835-1837.
- Perussia B, Fanning V, and Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat Immun Cell Growth Regul. 1985; 4: 120-137.
- Abb J, Zander H, Abb H, Albert E, Deinhardt F. Association of human leucocyte low responsiveness to inducers of interferon alpha with HLA-DR 2. Immunology. 1983; 49: 239-244.
- Fitzgerald-Bocarsly P. Human natural interferon-alpha producing cells. Pharmacol Ther. 1993; 60: 39-62.
- García-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in détente. Science. 2006; 312: 879-882.
- Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000; 192: 219-226.
- Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001; 2: 1144-1150.
- Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J Exp Med. 2011; 208: 2367-2374.
- Pagano L, Valentini CG, Pulsoni A, Fisogni S, Carluccio P, Mannelli F, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013; 98: 239-246.
- Feuillard J, Jacob MC, Valensi F, Maynadié M, Gressin R, Chaperot L, et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002; 99: 1556-1563.
- Dalle S, Beylot-Barry M, Bagot M, Lipsker D, Machet L, Joly P, et al. Blastic plasmacytoid dendritic cell neoplasm: is transplantation the treatment of choice? Br J Dermatol. 2010; 162: 74-79.
- Dietrich S, Andrulis M, Hegenbart U, Schmitt T, Bellos F, Martens UM, et al. Blastic plasmacytoid dendritic cell neoplasia (BPDC) in elderly patients: results of a treatment algorithm employing allogeneic stem cell transplantation with moderately reduced conditioning intensity. Biol Blood Marrow Transplant. 2011; 17: 1250-1254.
- Bueno C, Almeida J, Lucio P, Marco J, Garcia R, de Pablos JM, et al. Incidence and characteristics of CD4(+)/HLA DRhi dendritic cell malignancies. Haematologica. 2004; 89: 58-69.
- Chaperot L, Bendriss N, Manches O, Gressin R, Maynadie M, Trimoreau F, et al. Identification of a leukemic counterpart of the plasmacytoid dendritic cells. Blood. 2001; 97: 3210-3217.
- Jacob MC, Chaperot L, Mossuz P, Feuillard J, Valensi F, Leroux D, et al. CD4+ CD56+ lineage negative malignancies: a new entity developed from malignant early plasmacytoid dendritic cells. Haematologica. 2003; 88: 941-955.
- Kharfan-Dabaja MA, Lazarus HM, Nishihori T, Mahfouz RA, Hamadani M. Diagnostic and therapeutic advances in blastic plasmacytoid dendritic cell neoplasm: a focus on hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013; 19: 1006-1012.
- Pemmaraju N, Thomas DA, Kantarjian H. Analysis of outcomes of patients (pts) with blastic plasmacytoid dendritic cell neoplasm (BPDCN). J Clin Oncol. 2012; 30.
- Reimer P, Rüdiger T, Kraemer D, Kunzmann V, Weissinger F, Zettl A, et al. What is CD4+CD56+ malignancy and how should it be treated? Bone Marrow Transplant. 2003; 32: 637-646.