
Case Report
Ann Hematol Oncol. 2015; 2(10): 1066.
A Case of Non-Hodgkin’s Lymphoma Mimicking Cholangiocarcinoma Diagnosed by Endoscopic Ultrasound-Guided FNA
Ishikawa T and Belletrutti PJ*
Department of Medicine, University of Calgary, Canada
*Corresponding author: Paul J Belletrutti, Department of Medicine, Division of Gastroenterology and Hepatology, University of Calgary, Room 6D23, Research and Wellness Building, 3280 Hospital Drive Northwest, Calgary, T2N 4Z6, Alberta, Canada
Received: September 11, 2015; Accepted: November 28, 2015; Published: November 30, 2015
Abstract
Lymphoma is a rare cause of biliary obstruction and may mimic other malignant causes of jaundice. A 64-year-old man was admitted to hospital with painless jaundice and pruritus. Transabdominal ultrasound revealed biliary dilatation with a mass in the distal Common Bile Duct (CBD). CT and MRI confirmed intra- and extra hepatic biliary dilation, an abrupt caliber change in the CBD at the level of the mass in the porta-hepatis, and regional lymphadenopathy. Endoscopic retrograde cholangiogram demonstrated a severe stricture in the mid-CBD. Although brush cytology was negative for malignancy, the imaging findings favored a diagnosis of cholangiocarcinoma. Surgical resection was deferred due to the presence of lymphadenopathy. A biliary stent was placed and the patient was referred to our institution for a second attempt at a tissue diagnosis via Endoscopic Ultrasound (EUS)-guided Fine Needle Aspiration (FNA). The cytopathology combined with flow cytometry of the FNA specimen, however, was diagnostic of a large B-cell lymphoma. The patient was started on R-CHOP chemotherapy. EUS makes it possible to perform FNA of lesions that are inaccessible by other image-guided techniques. When FNA is performed, lymphoma should be considered as an alternate diagnosis, thus cytology should be combined with flow cytometry.
Keywords: Lymphoma; Endoscopic ultrasound; Flow cytometry
Case Presentation
A 64-year-old man was admitted to a regional hospital with painless jaundice and pruritus. He had noted dark colored urine, light colored stools and itchiness. He denied weight loss, fever, night sweats or loss of appetite. Past medical history included type-2 diabetes, hypertension, dyslipidemia, osteoarthritis and gastroesophageal reflux disease. He is an active smoker of 50 pack-years. He only drinks alcohol occasionally. He is a retired farmer but had no recalled exposure to environmental toxins or radiation. His family history is unremarkable for solid or hematological malignancies. Physical examination revealed jaundice but there were no palpable abdominal masses, hepatosplenomegaly or lymphadenopathy. He was afebrile and the remainder of his exam was unremarkable. Laboratory parameters included normal platelet count, WBC, hemoglobin, creatinine, calcium and total protein concentrations. However, total bilirubin was elevated at 206 umol/L (normal range <21) with ALP 382 U/L (normal range 55-170), GGT 1253 U/L (normal range 15- 90), LDH 228 umol/L (normal range 100-245) and ALT 181 U/L (normal range <60).
Transabdominal ultrasound demonstrated a hypoechoic mass centered on the distal Common Bile Duct (CBD) and significant upstream biliary dilation (Figure 1). CT and MR imaging both confirmed the findings of intra- and extra hepatic biliary dilation, an abrupt change in the caliber of the CBD at the level of the mass in the peri-portal region, and porta-hepatis lymphadenopathy (Figure 2). MR Cholangiopancreatography sequences demonstrated a tapering obstruction of the CBD with upstream dilation (Figure 3). Endoscopic Retrograde Cholangiogram (ERC) revealed a severe stricture in the mid-CBD (Figure 4). Intraductal brush specimens were obtained and sent for cytopathology and a plastic biliary stent was placed to relieve the obstruction. Although the cytology was nondiagnostic for malignancy, based on the presentation and imaging findings, the most likely diagnosis was felt be a cholangiocarcinoma. Surgical intervention was deferred due to the presence of regional lymphadenopathy. A repeat ERC was therefore performed and a permanent uncovered metal stent was placed for more reliable palliation. He was referred to our institution to obtain a pathological diagnosis with endoscopic ultrasound-guided FNA. On EUS, a 3cm well-defined hypoechoic mass was found in the periportal region more consistent with an enlarged lymph node as opposed to a CBD mass. Targeted FNA was performed of this lesion. A 25 gauge needle (Expect, M00550021, Boston Scientific, Natick, MA) was used and 3 separate specimens were obtained (Figure 5). Thin prep of the specimen showed scattered individual and aggregate lymphoid cells predominantly composed of atypical large cells (Figure 6). The atypical B-cells were positive for CD20 and CD45 (Figure 7). Partial expression of BCL2 by B-cells was also noted and the Ki67 proliferation index highlighted large atypical B-cells (Figure 7). Flow cytometric report indicated a lambda light chain restricted population with CD19/CD20/CD22 positive B lymphocytes aberrantly coexpressing CD10. The above results were in keeping with a B-cell neoplasm. The morphology and the immunoprofile of the neoplastic cells were consistent with a high grade B-cell lymphoma and the differential considerations were diffuse large B-cell lymphoma versus a high grade follicular lymphoma.
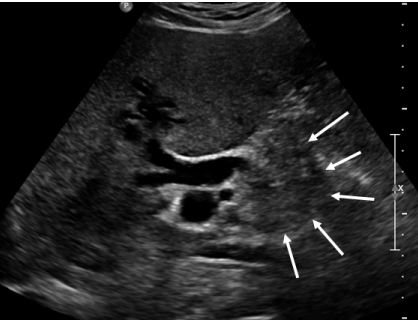
Figure 1: Transabdominal ultrasound shows a 3cm mass in the distal end
of the common bile duct (arrows) with dilation of the extra and intrahepatic
biliary tree.
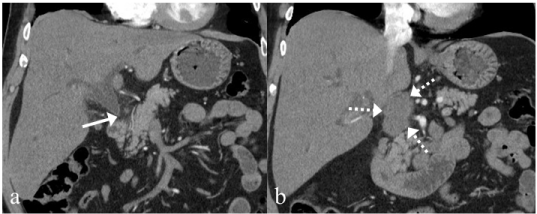
Figure 2: a: CT shows a rapid change in the caliber of the common bile duct
near the head of the pancreas (arrow). b: There is a lobulated low density
soft tissue mass in the peri-portal region which measures 3 X 5cm (broken
arrows). It abuts the inferior vena cava with mild mass effect.
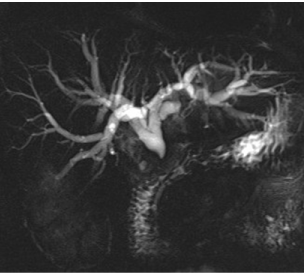
Figure 3: MRCP reveals acute termination of the extrahepatic duct at the
level of the distal common bile duct with intrahepatic biliary ductal dilation.
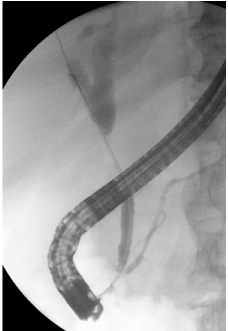
Figure 4: Endoscopic retrograde cholangiogram shows a severe stricture in
the middle of the common bile duct.
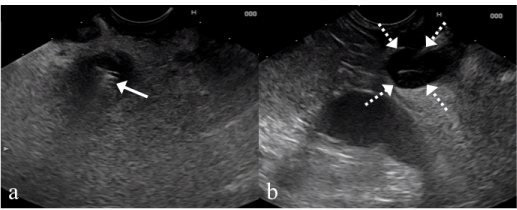
Figure 5: a: EUS reveals a metal stent (arrow) in the common bile duct with
surrounding hypoechoic tissue. b: A 3cm well-defined hypoechoic mass was
found in the peri-portal region just above the metal stent (broken arrow). FNA
was performed for this lesion.
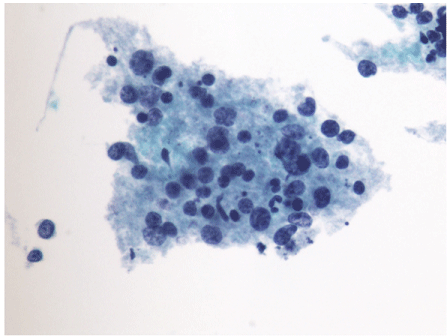
Figure 6: Thin Prep (liquid based cytology preparation, Papanicolaou stain)
of the specimen shows scattered individual and aggregates of lymphoid cells
predominantly composed of atypical large lymphoid cells and minority small
lymphoid cells in the background.
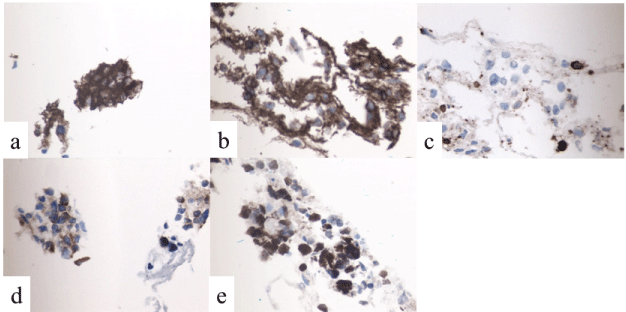
Figure 7: Immunohistochemical staining of the FNA specimens.
a: CD20 (positive), b: CD45 (positive), c: CD3 (negative), d: BCL2 (positive),
e: Ki67 (positive nuclear stain).
As both lymphomas are treated the same at our institution, no further tissue biopsy was required to refine the diagnosis. The CT scan of the neck, chest, abdomen and pelvis showed no pathologic lymph nodes except for those in the peri-portal region and bone marrow biopsy was normal. Therefore the final diagnosis was diffuse large B-cell lymphoma, differential diagnosis high-grade follicular lymphoma, International Prognosis Index (IPI) 2, Stage IVB. He was started on immunochemotherapy with R-CHOP. A CT scan after six cycles showed decrease in the size of porta-hepatis lymphadenopathy and FDG-PET indicated a complete metabolic response to therapy.
Discussion
The common symptoms of lymphoma are non-specific such as chills, fevers, weight loss or anorexia and it is rare to have obstructive jaundice. Approximately 1.3% of patients with lymphoma develop extra-hepatic biliary obstruction, with a frequency of 0.5% for Hodgkin’s lymphoma (HL) and 0.2%–2% for Non-Hodgkin’s lymphoma (NHL) [1-4]. In contrast, among all causes of malignant biliary obstruction, NHL is the cause in 1%–2% of patients [4,5]. However, lymphoma should always be considered in the differential diagnosis and biopsies should be performed in patients presenting with obstructing pancreatic, duodenal, or hepatobiliary masses because it is much more likely than other malignant causes of obstructive jaundice(namely cholangio- or pancreatic adenocarcinoma) to respond to chemotherapy [6].
In the present case, our patient presented with obstructive jaundice without any other specific findings and as imaging showed abnormalities only in the biliary tract and periportal region, he was misdiagnosed with cholangiocarcinoma. Although the bile duct brush cytology was negative for malignancy, based on imaging, surgery was ruled out and an uncovered metal stent was placed presuming a palliative treatment course. This type of biliary stent is considered permanent and cannot readily be removed. It is recommended that temporary plastic stents be used in patients who have obstructive jaundice at the time of lymphoma diagnosis because these strictures tend to quickly resolve with treatment [6]. One should always keep in mind the possibility of lymphoma in dealing with biliary obstruction and make an effort to obtain a tissue diagnosis before employing irreversible treatments, such as a permanent biliary stent.
Historically, excisional biopsies of affected lymph nodes, which permit examination of architectural pattern changes to further classify lymphoma subtypes, have been considered to be the gold standard for the diagnosis of lymphomas [7]. The role of FNA in the diagnosis of lymphoma has been controversial, with the exception of a few subtypes that can be reliably diagnosed solely by cytomorphological criteria [8]. The World Health Organization (WHO) and the Revised European-American Classification of Lymphoid Neoplasms (REAL) are modern lymphoma classifications that have been widely adopted by hematopathologists throughout the world [9]. The WHO/ REAL classification systems have incorporated criteria independent of tissue architecture in the subclassification of lymphomas. These criteria have included: (i) immunophenotyping to evaluate the presence of certain lymphoid markers on specific groups of lymphocytes; and (ii) flow cytometric cell surface marker analysis performed on fine-needle aspirates. This has emerged as an important ancillary technique for the evaluation of suspected lymphomas. These criteria have allowed EUS-FNA combined with Flow Cytometry (FC) to play an important role in the diagnosis of lymphomas.
To date, three studies have described the role of EUS-FNA for the diagnosis of lymphomas [10-12]. In all these studies, EUS-FNA cytology combined with FC showed high preoperative diagnostic accuracy. The overall sensitivity and specificity were 74-87% and 93- 100%, and Ribeiro et al. reported that the addition of FC to EUS-FNA permitted a statistically-significant increase in sensitivity from 44% to 86% [10].
Immunophenotyping by FC is a rapid and sensitive tool to evaluate for lymphoid markers. It can be applied to cytology specimens, because it only requires a small sample and can identify a population of abnormal cells that may not be apparent on cytology alone. The use of FNA cytology in the diagnosis of lymphoma has been greatly enhanced by FC, because it is useful in establishing monoclonality in B-cell lymphomas and in subclassifying lymphoma based on immunophenotypic markers [13]. FNA with FC is not ideally suited to diagnose HL and T-cell lymphomas. For HL, cytology can be negative if the classic Reed-Sternberg cells are lacking in the sample, and FC is generally of no added value because of the relative lack of neoplastic cells in relation to the background cells [14-16]. However, EUS-FNA with FC can be effectively used to diagnose NHL. As seen with various guided techniques of lymph-node FNA, the accuracy approaches 90% in the diagnosis of lymphoma [10-11]. In the present case, in addition to the immunohistochemical staining, FC showed CD19/CD20/CD22 positive B lymphocytes aberrantly co-expressing CD10, which led us to the diagnosis of a B cell neoplasm. Although the differentiation between diffuse large B-cell lymphoma and high grade follicular lymphoma was difficult, as both lymphomas are treated the same at our institution, he was able to start chemotherapy without further tissue acquisition.
In conclusion, we report a case of lymphoma which presented as obstructive jaundice and only EUS-FNA with FC yielded the correct diagnosis. Although obstructive jaundice is a rare complication of lymphoma, it must be distinguished from the more common malignancies that obstruct the biliary system because the treatment approach and response to systemic therapies is vastly different. EUS makes it possible to perform FNA of lesions that might be too small or inaccessible by other image-guided techniques, and when FNA is performed, cytology should be combined with FC to increase the chance for a definitive diagnosis.
References
- Odemis B, Parlak E, Basar O, Yuksel O, Sahin B. Biliary tract obstruction secondary to malignant lymphoma: experience at a referral center. Dig Dis Sci. 2007; 52: 2323-2332.
- Boddie AW Jr, Eisenberg BL, Mullins JD, Schlichtemeier AL. The diagnosis and treatment of obstructive jaundice secondary to malignant lymphoma: a problem in multidisciplinary management. J Surg Oncol. 1980; 14: 111-23.
- Di Sena V, Thuler FP, Macedo EP, Paulo GA, Della Libera E, Ferrari AP. Obstructive jaundice secondary to bile duct involvement with Hodgkin's disease: a case report. Sao Paulo Med J. 2005; 123: 30-32.
- Dudgeon DJ, Brower M. Primary chemotherapy for obstructive jaundice caused by intermediate-grade non-Hodgkin lymphoma. Cancer. 1993; 71: 2813-2816.
- Lokich JJ, Kane RA, Harrison DA, McDermott WV. Biliary tract obstruction secondary to cancer: management guidelines and selected literature review. J Clin Oncol. 1987; 5: 969-981.
- Ross WA, Egwim CI, Wallace MJ, Wang M, Madoff DC, Lee JH. Outcomes in lymphoma patients with obstructive jaundice: a cancer center experience. Dig Dis Sci. 2010; 55: 3271-3277.
- National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin's Lymphoma Pathologic Classification Project. Cancer. 1982; 49: 2112-2135.
- Hehn ST, Grogan TM, Miller TP. Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol. 2004; 22: 3046-3052.
- Jaffe ES, Harris NL, Diebold J, Muller-Hermelink HK. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. A progress report. Am J Clin Pathol. 1999; 111: S8-12.
- Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, Wang KK, Clain JE, Wiersema MJ. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001; 53: 485-491.
- Mehra M, Tamhane A, Eloubeidi MA. EUS-guided FNA combined with flow cytometry in the diagnoses of suspected or recurrent intrathoracic or retroperitoneal lymphoma. Gastrointest Endosc. 2005; 62: 508-513.
- Al-Haddad M, Savabi MS, Sherman S, McHenry L, Leblanc J, Cramer H, et al. Role of endoscopic ultrasound-guided fine-needle aspiration with flow cytometry to diagnose lymphoma: a single center experience. J Gastroenterol Hepatol. 2009; 24: 1826-1833.
- Noh KW, Wallace MB. Can EUS-guided FNA with flow cytometry be used to diagnose lymphoma? Gastrointest Endosc. 2005; 62: 514-516.
- Meda BA, Buss DH, Woodruff RD, Cappellari JO, Rainer RO, Powell BL, et al. Diagnosis and subclassification of primary and recurrent lymphoma. The usefulness and limitations of combined fine-needle aspiration cytomorphology and flow cytometry. Am J Clin Pathol. 2000; 113: 688-699.
- Dong HY, Harris NL, Preffer FI, Pitman MB. Fine-needle aspiration biopsy in the diagnosis and classification of primary and recurrent lymphoma: a retrospective analysis of the utility of cytomorphology and flow cytometry. Mod Pathol. 2001; 14: 472-481.
- Young NA, Al-Saleem TI, Ehya H, Smith MR. Utilization of fine-needle aspiration cytology and flow cytometry in the diagnosis and subclassification of primary and recurrent lymphoma. Cancer. 1998; 84: 252-261.