
Case Report
Ann Hematol Oncol. 2016; 3(1): 1072.
Brentuximab-AVD Treatment for a Patient with Hodgkin Lymphoma and Cystic Fibrosis
Petrini I¹*, Cervetti G², Galimberti S² and Cecconi N²
¹Department of Translational Medicine, Pisa University, Italy
²Department of Clinical and Experimental Medicine, Pisa University Hospital, Italy
*Corresponding author: Iacopo Petrini, Department of Translational Medicine, Pisa University, Italy
Received: January 08, 2016; Accepted: March 05, 2016; Published: March 10, 2016
Abstract
A 22-year-old man with cystic fibrosis developed Hodgkin lymphoma with diffuse localizations. The use of the standard treatment, ABVD and radiotherapy, could be harmful because the risk of pulmonary toxicity. Therefore, we adopted a modified ABVD schedule with brentuximab vedotin instead of bleomycin. After one year, 15 moths the patient was in complete remission without impairments of lung function. This observation supports the use brentuximab vedotin-AVD schedule in patients with lung diseases, for which bleomycin toxicity could be harmful.
Keywords: Brentuximab; Cystic fibrosis; Hodgkin lymphoma
Abbreviations
CT: Computed Tomography; FDG: Fluoro Deoxy Glucose; SUV: Standardized Uptake Values; IPS: International Prognostic Score; FVC: Forced Vital Capacity; FEV 1: Forced Expiratory Volume 1; MMEF: Maximum Midexpiratory Flow; BEACOPP: Bleomycin, Etoposide, Doxorubicin, Cyclophosphamide, Vincristine, Procarbazine and Prednisone; ABVD: Doxorubicin, Bleomycin, Vinblastine and Dacarbazine; GEV: Pegylated Liposomal Doxorubicin
Case Presentation
During November 2013, a 22-year-old man with cystic fibrosis was referred to our institution with a recent diagnosis of Hodgkin Lymphoma. Cystic fibrosis had been diagnosed earlier in the patient life with two heterozygous mutations: the mother’s allele carried the in-frame deletion of 3 nucleotides coding for a phenylalanine in 508 codon of the CTFR gene (ΔF508) whereas an uncommon and undetermined mutation was present in the father’s allele (molecular diagnosis from Galliera Hospital, Genova, in June 2000). During cystic fibrosis follow-up, a chest X-ray showed an irregular mediastinal profile and the computed tomography (CT) demonstrated a mass in the anterior mediastinum. The patient had mild breathing difficulties related to cystic fibrosis and Pseudomonas Aeruginosa was present in his sputum culture. The mass was removed by robotic surgery. The pathology report described a round-encapsulated tumor with a maximum diameter of 10 cm that was adherent to the parenchyma of the right superior lung lobe. The diagnosis was that of Hodgkin’s lymphoma: nodular sclerosis subtype, with areas of massive necrosis (CD30, PAX5 positive and CD15, CD20, CD3 and PAN-CK negative).
Baseline Fluorodeoxy Glucose (FDG) PET/CT showed hypercaptation in supradiaphragmatic lymph nodes and in the spleen: bilateral lateral cervical, retroclavicular, supraclavicular and mediastinal lymph nodes with maximum standardized uptake values (SUV) ranging from 3.5 to 10.7 and two focal lesions at the upper and lower pole of the spleen, SUV max 5.8. Blood counts, LDH, β2- microglubulin value, albumin levels and erythrocyte sedimentation rate were in the normal range. The patient did not refer any Hodgkin lymphoma related symptom. Therefore, the stage was IIIs A, according to the An Arbor classification with an International Prognostic Score (IPS) of 1 (sex male).
The treatment was decided thanking in consideration the risk of worsening lung fibrosis due to bleomycin that is present in the standard ABVD and BEACOPP schedules: the patient already had developed frequent episodes of pulmonary infections sustained by Pseudomonas Aeruginosa and impaired breathing capacity: forced vital capacity (FVC) 3400 (73%) forced expiratory volume 1 (FEV 1) 2640 (66%) FEV1/FVC 78%, maximum midexpiratory flow (MMEF) 2310ml/s (48%).
Initially, the patient received 2 cycles according to the IGEV regimen [1] without any hematological toxicity according to NIHCTCAE and without delay in schedule administration. However, the FDG-PET/CT documented the persistence of pathological lymphadenopathy (left latero cervical and supraclavicular), with an increase of the maximum standardize uptake values from 10.7 to 17 and a slight increase of node dimensions. Because of the response to chemotherapy was judged insufficient, a modified ABVD schedule was adopted with the replacement of bleomycin with brentuximab vedotin. The dose of brentuximab vedotin was 1.2 mg/kg every 14 days in combination with doxorubicin, dacarbazine and vinblastine. Prophylaxis was administered with daily fluconazole and twice a week sulfamethoxazole/trimethoprim for the entire duration of the treatment. A PET/CT was performed after 2 cycles of treatment showing a complete shutdown of FDG uptake. Patient received four additional cycles of AVD-brentuximab vedotin. The sixth cycle has been delayed of 1 week for an infective pulmonary complication: acute exacerbation of chronic obstructive pulmonary disease sustained by Staphylococcus Aureus that was successfully treated by tazobactam/ piperacillin and amikacin, according to antibiotic susceptibility test. Treatment toxicity included G3 neutropenia after the first and second cycles and a G1 neutropenia after the fifth cycle. The complete remission was confirmed after 1 month from the end of the treatment and currently maintained after 15 months follow-up. The respiratory function was preserved: FVC 4330 (93%) FEV 1 3200 (81%) FEV1/ FVC 83%, MMEF 2350 (48%) being all parameters comparable with those before the onset of the disease (Figure 1).
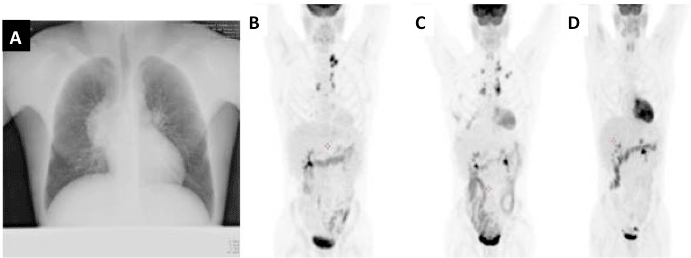
Figure 1: Patient’s imaging: (A) Chest Rx at time of the diagnosis; (B) PET scan after chest surgery; (C) PET scan after two cycles IGEV; (D) PET scan after two
cycles of Brentuximab AVD.
Discussion
The prognosis of patients with classical Hodgkin Lymphoma is improving during the last 20 years [2]. Unfortunately, these successes are limited by treatment toxicities [3]. Short-term toxicity is related to dose intensity and is relevant for aggressive schedules. Indeed, leukopenia and infections are common in patients during treatment. On contrary, long-term toxicities can arise after years and include second malignancies and heart and lung dysfunctions. In order to reduce short and long-term side effects aggressive chemotherapy is reserved for lymphomas with a poor prognosis and BEACOPP is currently the most active schedule [4]. BEACOPP prolongs PFS compared to ABVD, in patients with a high IPS, but carries a worse toxicity profile [5]. Modified ABVD schedules with only three chemotherapeutic agents are inferior to the use of the 4 drugs. Indeed, in the GHSG HD13 trial, modified ABVD without either decarbonize or bleomycin obtained inferior results compared to the full schedule [6]. A common bleomycin side effect is pulmonary fibrosis. In animal models, the bleomycin damage to basement membrane was induced by epithelial cell death, epithelial to mesenchymal transition and fibroblastic differentiation. At the biochemical level, chemokines, interleukins and the production of reactive oxygen species are responsible for bleomycin induced lung fibrosis [7]. In mice, the maximum lung fibrosis is obtained in 14 days after intratracheal instillation of bleomycin. However, 6 days after instillation of bleomycin early signs of fibrosis are already detectable [8]. In earlier studies, 53% of patients treated with ABVD developed respiratory symptoms. These symptoms usually regress with the discontinuation of therapy but 37% of patients have a decline in pulmonary function. After 6 ABVD cycles have been accomplished a progressive decline in FVC is observed until 15 months. However, it has been suggested that subsequently most patients recover their lung function. Interestingly, the baseline FVC, FEV1, and DLCO correlate with the impairment of FEV observed after therapy, suggesting that basal pulmonary diseases may increase the bleomycin toxicity [9].
Therefore, bleomycin should be used with caution in patients with impaired lung function especially in those with pulmonary fibrosis. In patients with Hodgkin lymphoma and concomitant pulmonary disorders alternative schedules without bleomycin but with a similar efficacy are advocated.
Brentuximab vedotin is a chimeric monoclonal anti-CD30 antibody conjugated with the antimitotic agent vedotin that has shown promising results in relapsed Hodgkin lymphoma [10]. Currently, several trials are evaluating brentuximab vedotin in relapsed/refractory Hodgkin lymphoma [11] and combination schedules with different chemotherapy regimens have been tested in phase I-II clinical trials [12]. Whereas the activity of brentuximab vedotin in association with ABV compared to classical ABVD is under investigation, the absence of significant toxicity of brentuximab vedotin-AVD has been already reported in phase I trial. However, not all the schedules including brentuximab vedotin have a favorable safety profile. Indeed, the schedule including brentuximab vedotin and ABVD is hampered by a relevant toxicity [13] and the combination of the anti CD30 antibody with gemcitabine, vinorelbine, Pegylated liposomal doxorubicin (GEV) has shown unacceptable lung toxicity leading to the premature closure of the trial [14].
Therefore, brentuximab vedotin-AVD could be an alternative treatment for patients with pulmonary dysfunction in order to avoid bleomycin. Brentuximab vedotin-AVD could be tested in patients with Hodgkin lymphoma and cystic fibrosis that spontaneously develop pulmonary insufficiency and recurrent infections. Indeed, patients with cystic fibrosis are fragile and careful considerations on safety are necessary for the choice of the most appropriate treatment.
Cystic fibrosis is the most frequent autosomal recessive disorder in Caucasian. Mutations of the CFTR gene (Fibrosis Transmembrane Conductance Regulator) that impair protein function cause cystic fibrosis. CFTR is an ABC-class transmembrane channel that conducts chloride and thiocyanate across the membrane of epithelial cells regulating the viscosity of mucous secretions. Over 15000 mutations have been reported in CFTR. Different mutations determine different degrees of protein dysfunction, which is reflected in the severity of the disease. A fluid mucous secretion is request for the proper function of several organs including lungs and pancreas. The viscous mucus secretions of patients with cystic fibrosis determine severe damage to the pancreas and lungs with consequent malabsorption, lung infections and fibrosis responsible for the breathing impairment. The disease evolves to a respiratory insufficiency that may lead to lung transplantation or death [15].
This case remarks that not all patients with Hodgkin lymphomas can receive standard chemotherapy. Bleomycin related lung-toxicity is harmful for patients with lung disorders especially for those with a preexisting pulmonary fibrosis. Moreover, chemotherapy toxicity includes leukopenia and neutropenia that predispose to infections, which can worsen the extension of reactive fibrosis. These considerations become extremely relevant for patients with cystic fibrosis because of the risk of a respiratory function decline and septic lung infections. Moreover, the patient described in this report had been colonized by Pseudomonas Aeruginosa, a common condition in cystic fibrosis, that further increase the risk of infection’s reactivation. Brentuximab vedotin is successfully used in relapsed Hodgkin lymphoma and is becoming a promising drug also in front line therapy for patients that require a schedule modification for the risk of toxicity. In this case report, we showed that brentuximab vedotin- AVD combination may be safely and successfully administered to a very frail patient After 15 months, there were not evidences of increased lung fibrosis or infections related to Pseudomonas Aeruginosa. In the absence of designed phase III clinical trial we suggest that patients affected by lung fibrosis could be candidate to brentuximab vedotin-AVD schedule.
References
- Santoro A, Magagnoli M, Spina M, Pinotti G, Siracusano L, Michieli M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin's lymphoma. Haematologica. 2007; 92:35-41.
- Armitage JO. Early-stage Hodgkin's lymphoma. N Engl J Med. 2010; 363: 653-662.
- Aleman BM, van den Belt-Dusebout AW, Klokman WJ, Van't Veer MB, Bartelink H, van Leeuwen FE. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol. 2003; 21: 3431-3439.
- Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003; 348: 2386-2395.
- Merli F, Luminari S, Gobbi PG, Cascavilla N, Mammi C, Ilariucci F, et al. Long-Term Results of the HD2000 Trial Comparing ABVD Versus BEACOPP Versus COPP-EBV-CAD in Untreated Patients With Advanced Hodgkin Lymphoma: A Study by Fondazione Italiana Linfomi. J Clin Oncol. 2015.
- Behringer K, Goergen H, Hitz F, Zijlstra JM, Greil R, Markova J, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin's lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2015; 385 :1418-1427.
- Della Latta V, Cecchettini A, Del Ry S, Morales MA. Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol Res. 2015; 97: 122-130.
- Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol. 2002; 83:111-119.
- Hirsch A, Vander Els N, Straus DJ, Gomez EG, Leung D, Portlock CS, et al. Effect of ABVD chemotherapy with and without mantle or mediastinal irradiation on pulmonary function and symptoms in early-stage Hodgkin's disease. J Clin Oncol. 1996; 14:1297-305.
- Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012; 30: 2183-2189.
- Bartlett NL, Chen R, Fanale MA, Brice P, Gopal A, Smith SE, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014; 7:24.
- Michallet AS, Guillermin Y, Deau B, Lebras L, Harel S, Amorin S, et al. Sequential combination of gemcitabine, vinorelbine, pegylated liposomal doxorubicin and brentuximab as a bridge regimen to transplant in relapsed or refractory Hodgkin lymphoma. Haematologica. 2015; 100: e269-71.
- Younes A, Connors JM, Park SI, Fanale M, O'Meara MM, Hunder NN, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013; 14: 1348-1356.
- Blum KA, Jung SH, Johnson JL, Lin TS, Hsi ED, Lucas DM, et al. Serious pulmonary toxicity in patients with Hodgkin's lymphoma with SGN-30, gemcitabine, vinorelbine, and liposomal doxorubicin is associated with an FcgammaRIIIa-158 V/F polymorphism. Ann Oncol. 2010; 21: 2246-2254.
- O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009; 373: 1891-1904.