
Case Report
Ann Hematol Oncol. 2016; 3(1): 1073.
Non-Hodgkin Lymphoma of the Tongue: New Case Report and Review of the Literature
Alami EL¹*, Azami MA², Bourhafour I³, Boukir A4, Ichou M¹ and Errihani H4
¹Departments of Medical Oncology, Military Hospital Med V, Morocco
²Department of Pathology, Military Hospital Mohamed V, Morocco
³Department of Radiotherapy, National Institute of Oncology, Morocco
4Department of Medical Oncology, National Institute of Oncology, Morocco
*Corresponding author: EL Alami, Departments of Medical Oncology, Military Hospital Med V, Morocco
Received: January 25, 2016; Accepted: March 05, 2016; Published: March 10, 2016
Abstract
Primary non-Hodgkin’s lymphoma (NHL) of the oral region is rare. Oral manifestations are present in 3-5% of cases of NHL.We describe a case of 70 year old female who presented with a mass lesion of tongue that was diagnosed as diffuse large B cell lymphoma. The patient was treated with 4 RCHOP chemotherapy (Rituximab 375 mg/ m2, cyclophosphamide 750 mg/ m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/ m2 and prednisone 100 mg/day during 5 days; the protocol is repeated every 3 weeks) and radiotherapy (36 Gy in 20 fractions). The lesion was completely disappeared after a first cycle. She has currently remained disease free for 6 months.
Keywords: NHL; R-CHOP; Tongue
Abbreviations
NHL: Non-Hodgkin Lymphoma; R-CHOP: Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisone; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography; FDG: 18F-Fluorodeoxyglucose; BR: Bendamustine and Rituximab
Introduction
Lymphomas are malignant neoplasms of the lymphocyte cell lines. They mainly involve lymph nodes, spleen and other nonhematopoietic tissues. They are mainly classified as either Hodgkin’s or non-Hodgkin’s Lymphoma (NHL), and of either B-lymphocyte or T-lymphocyte origin.
Extra nodal lymphomas represent 20-30% of non- Hodgkin’s lymphoma (NHL), the head and neck is the second most common region for the extra-nodal lymphomas after that of gastrointestinal tract. The oral manifestations are seen in 3-5% of cases of NHL.
In this paper, we will present a case of female patient treated for non Hodgkin en lymphoma of the right lateral border of the tongue and has been successfully managed by chemotherapy and radiotherapy.
Case Presentation
A 70 year old woman, presented with a 6 month history of throat pain radiating to the right ear. The evolution was marked by the appearance of a mass of the tongue associated with a shortness of breath, dysphagia and difficulty of mastication and speech. No weight loss, night sweats or fever was reported. Her medical history was unremarkable. No medications were taken on a regular basis and the patient was a non-smoker.
Oral examinations by observation show asymmetry of the tongue. Digital palpation revealed a large sub-mucosal mass involving the right lateral border of the tongue. There was no ulceration or superficial growth on the surface of the tongue.
We note a cervical lymph-adenopathy in contralateral of the tumor, however no abdominal organomegaly was noted and the remainder of the physical examination was unremarkable (Figure 1).
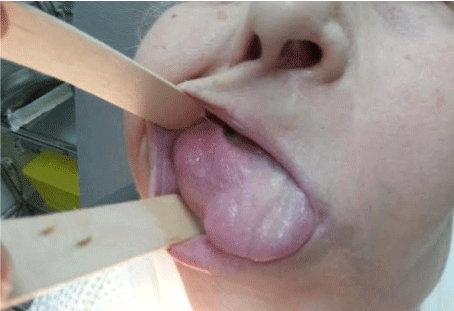
Figure 1: Lesion on lateral border of tongue (before Chemotherapy).
A Magnetic Resonance Imaging (MRI) revealed a raised mass on the right lateral border of the tongue (Figure 2). We had seen a left cervical lymphadenopathy.
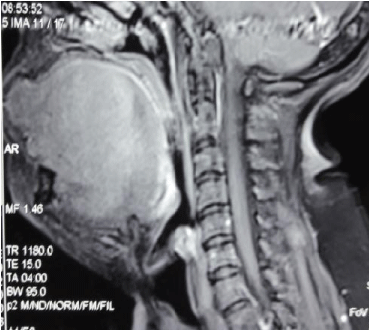
Figure 2: Sagittal view of the lesion at the MRI.
There was no thoracic, abdominal or pelvic lesion in the CT scan. Bone marrow biopsy showed no abnormality. A complete blood count and routine blood chemistry were normal (Lactate Dehydrogenase 190 U/l, WBC 4, 5 μ/L, hemoglobin, 13.5g/dL, and platelet count 200,000 μ/L). Serology for human immunodeficiency virus was negative.
A biopsy was performed. The histological examination revealed diffuse infiltration by malignant lymphoma with medium to large size lymphocytes a diagnosis of primary B cell lymphoma of the tongue was made based on findings of monotonous infiltration by large lymphoid cells with irregular nuclear membrane and prominent nucleoli, surrounding and entrapping the mucous glands and the musculature of the tongue (Figure 3). Immunohistochemical testing was positive for leukocyte common antigen and CD20 (Figure 4, 5).
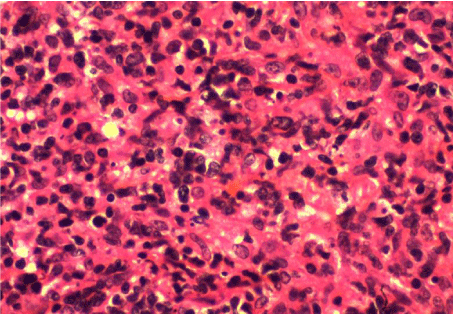
Figure 3: Pathologic finding of tongue mass area biopsy showing atypical
lymphoid cell proliferation (H&E, ×400).
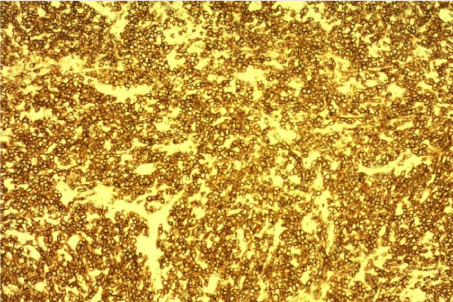
Figure 4: CD20 immunostain of diffuse large cell B cell lymphoma of the
tongue.
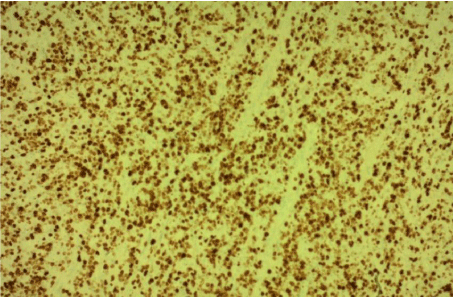
Figure 5: KI 67 immunostain of diffuse large cell B cell lymphoma of the
tongue.
The patient was treated with chemotherapy and external beam radiation (36 Gy).
Four cycles of R-CHOP regimen (Rituximab 375 mg/m², cyclophosphamide 750 mg/m², doxorubicin 50 mg/m², vincristine 1.4 mg/m² and prednisone 100 mg/day during 5 days; the protocol is repeated every 3 weeks) , The treatment was followed by external beam radiotherapy (36 Gy in 20 fractions). We note a clinical resolution of the mass after a first cycle (Figure 6). He has currently remained disease free for 6 months.
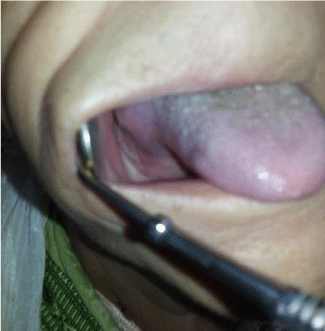
Figure 6: Regression of lesion (After a first cycle of chemotherapy).
Discussion
Malignant lymphoma of the oral cavity is rare [1], the extranodal site was the primary site of disease in 20-30% of cases; in particular, the most affected areas are the gastroenteric tract and the head-neck area (in descending order) [2].
The intra-oral location is not frequent (3-5% of cases) [3] and the most affected sites are tonsils (55% of oral cases), palate (30% of cases) and genial mucosa (2% of cases). There are, instead, sporadic manifestations affecting the tongue (2% of cases), the buccal floor (2% of cases) and the retromolar trigone (2% of cases) [4].
Primary malignant lymphoma of the tongue affects the elderly, especially over the 6th decade of life [5], and the male / female ratio was 2/1 [6].
The most common symptoms are local swelling, pain and discomfort in the throat or ulceration. The tumor may manifest as a submucosal mass, a polypoid bulky mass with a smooth mucosal surface, or as an ulcerated lesion. Involvement of the intrinsic tongue musculature causes restriction of movement, dysarthria and dysphagia [5]. Occasionally, the tumor may cause upper airway obstruction.
Little is known about the etiologic factors for primary lymphomas involving the oral cavity. However the oral plasmablastic lymphomas have been reported in association with the acquired immune deficiency syndrome [7,8] in our case the patient was HIV negative.
Computed tomography of the head and neck, chest, abdomen and pelvis is the mainstay of staging for oropharyngeal lymphoma as well as other nodal lymphomas. Bone marrow biopsy is equally mandatory for staging. Concurrent Positron Emission Tomography (PET) with 18F-fluorodeoxyglucose (FDG) and computed tomography (PET/ CT) is a useful method for staging and assessment of therapeutic response [9].
Treatment of primary extranodal lymphomas of the tongue is not clearly defined [10], although it has been suggested a chemotherapy followed by involved field radiotherapy for patients with early stage diffuse large-cell lymphoma [11,12]. Treatment recommendation for diffuse large B-cell lymphoma is generally identical for nodal and extra nodal diseases [13]. The standard treatment for patients with above the level of stage II diffuse large B-cell lymphoma except primary extranodal organ non-hodgkin lymphoma had been combination chemotherapy with CHOP. The addition of monoclonal antibody to CD20 (rituximab) to CHOP has been found to improve the complete response rate and prolongs the event-free and overall survival in patients with diffuse large B-cell lymphoma, without a clinically significant increase in toxicity [14,15] .
The randomized, non inferiority (NI), global phase III BRIGHT study evaluated the efficacy and safety of bendamustine plus rituximab (BR) vs a standard [R-CHOP] or rituximab plus cyclophosphamide, vincristine, and prednisone [R-CVP]) for treatment naïve patients with indolent non-Hodgkin’s lymphoma or mantle cell lymphoma, the combination of bendamustine and rituximab (BR) has been shown to be a well-tolerated and an active treatment regimen [16].
Conclusion
NHL of the tongue is very rare and all reported studies include a small number of patients. Treatment recommendation is generally identical for nodal and extranodal diseases. The standard treatment of localized stage was the chemotherapy with R-CHOP followed by radiotherapy. We report another case of the literature that can be successfully managed by this strategy.
References
- Dey P, Luthra UK, Sheikh ZA, Mathews SB. Fine needle aspiration cytology of primary non-Hodgkin's lymphoma of the tongue. A case report. Acta Cytol. 1999; 43: 422-4.
- Clearly KR, Batsakis JG: Sinonasal lymphomas. Ann Otol Rhinol Laryngol. 1994; 103: 911-914.
- Vaswani B, Shah M, Shah PM, Parikh BJ, Anand AS, Sharma G. Non Hodgkin’s Lymphoma of Tongue - A Case Report. Indian Journal of Medical & Paediatric Oncology. 2008; 29: 59-61.
- Regezi JA, Sciubba JJ: Patologia orale, dalla diagnosi alla terapia. Terza edizione. Delfino editore 2001.
- Maheshwari G, Baboo HA, Shah NM, Patel MH, Shah R. Primary non-Hodgkin's lymphoma of the oral tongue. Turk J Cancer. 2001; 31:121-124.
- Terada T. Primary non-Hodgkin B-cell lymphoma of the tongue. Br J Oral Maxillofac Surg. 2011; 49: e18-9.
- Lim JH, Lee MH, Lee MJ, Kim CS, Lee JS, Choi SJ, et al. Plasmablastic Lymphoma in the Anal Canal. Cancer Res Treat. 2009; 41:182-185.
- Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, et al. Plasmablastic lymphomas of the oral cavity: A new entity associated with the human immunodeficiency virus infection. Blood. 1997; 89: 1413-1420.
- Elstrom RL, Leonard JP, Coleman M, Brown RK. Combined PET and low-dose, noncontrast CT scanning obviates the need for additional diagnostic contrast-enhanced CT scans in patients undergoing staging or restaging for lymphoma. Ann Oncol. 2008; 19: 1770-1773.
- Guastafierro S, Falcone U, Celentano M, Cappabianca S, Giudice A, Colella G. Primary mantle-cell non-Hodgkin’s lymphoma of the tongue. Int J Hematol. 2008; 88: 206-208.
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346: 235-242.
- Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998; 339: 21-26.
- You JY, Chi KH, Yang MH, Chen CC, Ho CH, Chau WK. Radiation therapy versus chemotherapy as initial treatment for localized nasal natural killer (NK)/T-cell lymphoma: A single institute survey in Taiwan. Ann Oncol. 2004; 15:618-625.
- Marcus RW, Sweetenham JW, Williams ME. Lymphoma: Pathology, Diagnosis and Treatment. Cambridge University Press. 2007.
- Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005; 23: 5027-5033.
- Flinn IW, Van der Jagt R, Kahl BS, Wood P, Hawkins TE, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014; 123: 2944-2952.