
Research Article
Ann Hematol Oncol. 2016; 3(2): 1078.
Granulocytic Myeloid Derived Suppressor Cells are Increased in Multiple Myeloma and can Predict outcome in Patients Treated upfront with Lenalidomide
Romano A*, Parrinello NL, La Cava P, Tibullo D, Giallongo C, Parisi M, Vetro C, Conticello C and Di Raimondo F
Division of Hematology, University of Catania, Italy
*Corresponding author: Alessandra Romano, Division of Hematology, University of Catania, UOC Hematology with Bone Marrow Transplantation, Ferrarotto Hospital, Via Citelli 6, 95124 Catania, Italy
Received: March 16, 2016; Accepted: May 02, 2016; Published: May 04, 2016
Abstract
Background: Recent studies have indicated a role for the myeloid compartment in the biology of Multiple Myeloma (MM) and the neutrophil to lymphocyte ratio (NLR) is emerging predictors of progression free survival (PFS) and overall survival (OS) in MM. In addition, Myeloid-Derived Suppressor Cells (MDSC), a heterogeneous population of myeloid cells with peculiar immunosuppressive properties against T-cells, are increased in MM, as recently described.
Aim: We investigated MDSC and clinical variables at diagnosis (including NLR) and their impact on outcome of patients treated upfront with novel agents (Lenalidomide and bortezomib).
Methods: We evaluated by flow cytometry G-MDSC (CD11b+CD33+CD14- HLADR-) in 45 newly diagnosed MM patients treated upfront with lenalidomide (N=30) or bortezomib (N=15), compared to 50 MGUS and 30 healthy subjects matched for sex and age.
Results: G-MDSC percentage in MM whole blood was greater than healthy controls (61.2±1.6% versus 51.7±1.4%, p< 0.001) with a large overlapping with granulocytes.
G-MDSC were higher in presence of extensive bone disease (p= 0.02), in patients carrying LDH ≥2 UPN (p= 0.0025) and fibrinogen ≥40 mg/dL (p= 0.01). G-MDSC were also positively correlated with neutrophil to lymphocyte ratio (NLR) recently described as a novel biomarker in MM (r2=0.60, p< 0.0001).
G-MDSCwb was reduced after exposure to lenalidomide-based regimen (p< 0.0001) but not to bortezomib. Patients treated upfront with lenalidomide and G-MDSCwb >60% at diagnosis had shorter progression free survival (PFS) than those with less than 60% (16.8 versus 47.0 months, p= 0.04).
Conclusion: G-MDSC is increased in MM; they can predict response to lenalidomide. NLR could be surrogates for G-MDSC and this can explain the recently reported prognostic meaning.
Keywords: Multiple myeloma; G-MDSC; MGUS
Introduction
Multiple myeloma (MM) is the second most frequent hematological neoplasia, characterized by the accumulation of malignant plasma cells within the marrow microenvironment leading to variable anemia, bone pain, renal impairment, hypercalcemia and infections [1,2].
Monoclonal gammopathy of undetermined significance (MGUS) is an asymptomatic, pre-malignant disorder characterized by monoclonal plasma cell proliferation in the bone marrow and absence of end-organ damage [3].
Virtually all cases of MM arise from MGUS through a multi-stage process of proliferation and dissemination [4-6]. MGUS plasma cells do not exhibit clear genetic and/or phenotypic markers distinguishing from MM [7-9], and it is still not possible to predict MGUS progression to MM in individual patients, with a heterogeneous average rates of progression as low as 0.26% and as high as 12% per year [5].
While in MGUS T-cells isolated from the bone marrow are able of mounting vigorous response against autologous pre-malignant cells, this phenomenon is not observed in MM [10]. On the contrary, in MM the immune function is impaired as consequence of cellular defects and represents an immunologically “permissive” microenvironment [11]. Several mechanisms may concur in the reduction of immunesurveillance in MM [12,13] and in this perspective, the presence of myeloid derived suppressor cells (MDSC) has been recently described in mouse models [14,15] and human MM [14,16,17].
MDSC are a heterogeneous population of myeloid derived cells [18] that can utilize multiple pathways to elicit tumor immune-editing, including secretion of immunosuppressive cytokines, up-regulation of reactive oxygen species, and T-cell function impairment through perturbation of the arginine metabolism [19]. In tumor-bearing mice two main MDSC populations have been distinguished based on their immunophenotype and morphology: monocytic MDSCs (mo-MDSC) identified as CD11b+CD115+Ly6-G-Ly6-Chigh, and granulocytic MDSCs (G-MDSC) with the phenotype CD11b+CD115– Ly6G+Ly6Clow, which represent up to 75% of all MDSC, while the identification and isolation of human MDSC subsets are challenging due to the heterogeneous characteristics [20,21] and for G-MDSC the overlapping immunophenotype with granulocytes circulating in peripheral blood [22-27].
MDSC are increased in MM [16] and most recent reports indicate that the G-MDSC subpopulation could play a role in the biology of disease [14,17]. However, its clinical impact has never been elucidated.
Thus, we investigated the clinical impact of G-MDSC in MGUS and in newly diagnosed MM patients undergoing treatment with novel agents.
Methods
Patients and controls
Between January 2010 and December 2012, G-MDSC was investigated in 45 newly diagnosed MM and 50 MGUS patients (Table 1). Patients were devoid of immune-mediated diseases and acute or chronic viral infections (based on routinely clinical assessment for TORCH agents and replicative status of HAV, HBV, HCV and HIV viruses) to exclude any interference on immune-regulatory mechanisms. All MM patients had measurable disease, defined as a serum-M protein level of at least 1g per deciliter, a urine-M protein level of at least 200 mg per day, or both according to International Myeloma Working Group Criteria [28]. All MGUS patients had a stable condition with at least 2 years of follow up. Thirty healthy subjects (age 35-60 years) were recruited in the study as controls.
MGUS
N=50 (percentage)
MM
N=45 (percentage)
MM treated upfront with lenalidomide
N=30 (percentage)
MM treated upfront with bortezomib
N=15 (percentage)
Median age (range)
57 (49-70)
60 (42-88)
59 (42-88)
60 (52-71)
Males/Females
21/29
28/17
18/12
10/5
Istotype, n (%)
IgG
30 (60)
30 (68)
23 ()
7 ()
IgA
17 (34)
8 (18)
5 ()
3 ()
IgM
3 (6)
1 (2)
0 (0)
1 ()
k- λ
0 (0)
6 (12)
2 ()
4 ()
Cytogenetics, n (%)
Normal
8 (16)
16 (36)
13 ()
3 ()
del 13
17 (34)
5 (11)
2 ()
3 ()
del 17
1 (2)
6 (13)
4 ()
2 ()
t (4;14)
6 (12)
10 (22)
6 ()
4 ()
not performed/failed
18 (36)
8 (18)
5 ()
3 (0)
Hemoglobin, g/l (range)
13 (12-14.5)
10.6 (6.5-14.8)
10.6 (6.5-14.8)
10.7 (7.5-13.8)
Platelets 1000/uL (range)
219 (180-315)
221 (90-384)
221 (90-384)
221 (90-384)
WBC1000/uL (range)
5.7
5.2 (4.7-10.5)
5.2 (4.7-10.5)
5.0 (4.9-10.2)
ALC.1000/uL (range)
2.1 (0.3-4.2)
1.9 (1.0-3.2)
1.9 (1.0-3.6)
1.9 (1.2-3.2)
Bone marrow plasmocytosis>50 %, n (%)
0
34 (30-90)
30 (30-90)
34 (30-70)
C-reactive protein, mg/L (range)
0.1(0.01-3.5)
0.4 (0.01-7.5)
0.4 (0.01-7.5)
0.4 (0.01-7.5)
LDH, U/l (range)
195 (103-215)
209 (109-708)
210 (109-700)
209 (115-708)
Beta-2 microglobulin, mg/L
2.8 (1.8-3.2)
4.1 (1.9-9.9)
4.1 (1.9-9.9)
4.1 (1.9-9.9)
Serum Albumin, g/dL (range)
3.91 (3.2-4.2)
3.9 (2.5-4.5)
3.9 (2.5-4.5)
3.9 (2.5-4.5)
GFR, mL/min
80 (76-110)
70 (16-90)
72 (46-90)
69 (16-90)
ESR , mm/h
15 (0-20)
72 (6-134)
60 (6-134)
82 (16-134)
STAGE ISS
1
N.A.
8 (18)
5 ()
3 ()
2
N.A.
19 (42)
10 ()
9 ()
3
N.A.
18 (40)
15 ()
3 ()
Table 1: Patients’ characteristics.
Thirty MM patients received four 28-days cycles of lenalidomide 25 mg daily on days 1 to 21 and dexamethasone 40 mg daily on days 1, 8, 15, 22 (Rd); the remaining 15 MM patients received 21-days cycles of bortezomib 1.3 mg/m2 and dexamethasone 40mg daily on days 1, 4, 8, 11 (VD) associated or not to adriamicina (PAD) as part of their induction therapy. All eligible patients underwent autologous stem cell transplantation.
White blood cell count and types (neutrophils and lymphocytes) were determined by electrical impedance method in automatic blood counter device (Beckman Coulter LH 750). The neutrophil to lymphocyte ratio (NLR) calculated using data obtained from CBC.
Baseline characteristics of evaluated patients are listed in (Table 1). Median age in MM group was 60 (range 42-88), 40% patients were stage III according to ISS classification. Cytogenetics was available for 37 (82%) patients, and it was abnormal in 21/37 patients.
Reagents
The following anti-human antibodies were purchased from Beckman Coulter: CD11b FITC (clone bear1), CD33 PE (clone D3HL60.251), CD14 PC5 (clone RMO52), HLA-DR- ECD (Clone Immu-357), HLA-DR- PC5 (Clone Immu-357), CD3 ECD (clone UCHT1), CD69 PE (clone TP1.55.3), CD71 FITC (clone DYJ1.2.2).
Fluorescent-labeled isotype matched control antibodies were also purchased from Beckman Coulter, used as negative controls.
G-MDSC evaluation
EDTA whole blood sample (50 μL) was stained with monoclonal antibodies (mAbs, 10 μL for each) and respective isotypic controls. Using sequential gating strategy, G-MDSC cells were identified as CD11b+CD33+CD14-HLADR- in whole blood (G-MDSC) or in the fraction of mono nucleated cells obtained after ficoll separation (G-MDSC-ficoll). The acquisition and analysis was performed with a Beckman Coulter FC-500 flow cytometer (10,000 cells were analyzed).
Results were expressed as percentages of leukocytes for G-MDSC and correlated to clinical findings: burden disease (stage ISS, albumin, beta-2 microglobulin, number of osteolytic lesions, LDH, bone marrow plasma cell infiltration); inflammation parameters (fibrinogen, ESR, C-RP); cytogenetics; NLR.
Statistical methods
Descriptive statistics were generated for analysis of results and p-value under 0.05 was considered significant. Qualitative results were summarized in counts or percentages. Data were plotted as mean±Standard Error Mean (SEM) or using boxes and whiskers at 5°-95° percentile. Comparisons were performed using the unpaired t-test or Mann-Whitney test for immunosuppressive assays. Association among variables was evaluated by linear regression.
Receiver operating characteristic (ROC) analysis was used to determine the sensitivity and specificity of G-MDSC count and to identify a cut-off to discriminate between patients relapsed or not. This threshold was then carried forward to predict progression free Survival (PFS).
Data were elaborated using GraphPad Prism version 6.00 for Windows, GraphPad Software, San Diego California USA, www. graphpad.com.
Results
G-MDSC and clinical variables in newly diagnosed MM
GMDSC were evaluated at diagnosis in MM patients and in MGUS subjects with a stable condition from at least 5 years. G-MDSC were evaluated as percentage of whole blood (G-MDSCwb), with a progressive increase from healthy controls (51.7±1.4%) versus MGUS (56.2±1.2, p= 0.01) versus MM 61.2±1.6 (p= 0.026, Figure 1A).
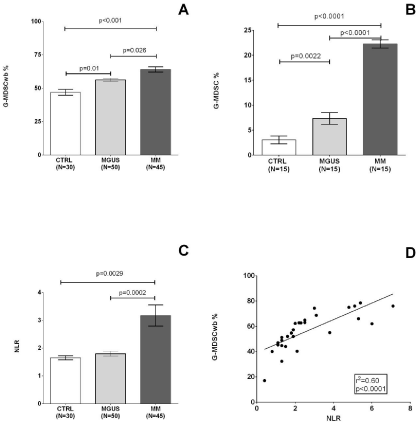
Figure 1: G-MDSC in peripheral blood of MGUS/MM and correlation with NLR and LMR.
Percentage of G-MDSC in whole peripheral blood (G-MDSCwb, A) and in PBMC (B), NLR (C) from CBC are shown for healthy subjects, MGUS and MM patients
at diagnosis.
Association between G-MDSC percentage and NLR is shown for MM patients respectively in panel D.
G-MDSC in peripheral blood of MGUS/MM and correlation with NLR and LMR.
Percentage of G-MDSC in whole peripheral blood (G-MDSCwb, A) and in PBMC (B), NLR (C) from CBC are shown for healthy subjects, MGUS and MM patients
at diagnosis.
Association between G-MDSC percentage and NLR is shown for MM patients respectively in panel D.
Abbreviations: CBC: Complete Blood Count; G-MDSC: granulocyte-like subset of MDSC; G-MDSCwb: granulocyte-like subset of MDSC in whole peripheral
blood; NLR: Neutrophil to Lymphocyte Ratio. CBC: Complete Blood Count; G-MDSC: granulocyte-like subset of MDSC; G-MDSCwb: granulocyte-like subset of MDSC in whole peripheral
blood; NLR: Neutrophil to Lymphocyte Ratio.
G-MDSCwb were higher in presence of extensive bone disease (at least 3 lesions at the X-ray scan, respectively 45.9±8.6% versus 60.2±1.9%, p= 0.02, in patients carrying LDH ≥2 UPN (53.7±2.2% versus 66.8±3.4%, p= 0.0025) and fibrinogen ≥40 mg/dL (51.8±3.4% versus 62.9±2.2%, p= 0.01, Table 2). The difference in the amount of MDSC in MM as compared to MGUS and healthy controls was more evident when we evaluated G-MDSC as percentage of PBMC (G-MDSC-ficoll) (22.2±0.8, versus 7.3±1.1, versus 3.1±0.7 respectively, p< 0.0001, Figure 1B).
Clinical variable
G-MDSC
Mean ± SEM
p-value
ISS stage 1-2
57.9 ± 2.8
0,97
ISS stage 3
57.7 ± 3.0
Albumin < 3.5 g/dL
60.8 ± 4.2
0,41
Albumin ≥ 3.5 g/dL
56.7 ± 2.4
Beta-2 microglobulin < 5.5 g/dL
57.3 ± 2.6
0,71
Beta-2 microglobulin ≥ 5.5 g/dL
59.1 ± 3.5
Bone lesions 0-2
45.9 ± 8.6
0,02
Bone lesions > 2
60.2 ± 1.9
LDH < 2UPN
53.7 ± 2.2
0,002
LDH ≥ 2 UPN
66.8 ± 3.4
Fibrinogen < 450 mg/dL
51.8 ± 3.4
0,01
Fibrinogen ≥ 450 mg/dL
62.9 ± 2.2
ESR < 40mm/hour
58.5 ± 3.8
0,85
ESR ≥ 40mm/hour
57.5 ± 2.6
C-RP <5 mg/L
58.4 ± 3.7
0,83
C-RP ≥ 5 mg/L
57.5 ± 2.5
Normal cytogenetics
55.8 ± 2.5
0,92
Abnormal cytogenetics
56.3 ± 2.2
NLR < 2
46.7 ± 1.9
<0,0001
NLR ≥ 2
66.0 ± 1.5
LMR > 2.9
55.1 ± 2.0
0,01
LMR ≤ 2.9
66.5 ± 3.4
Plasma cells in bone marrow <50%
55.5 ± 3.4
0,35
Plasma cells in bone marrow ≥ 50%
59.7 ± 2.4
GFR < 40mm/hour
59.7 ± 3.8
GFR ≥ 40mm/hour
55.4 ± 2.6
0,85
Table 2: G-MDSC and clinical variables in MM.
Since G-MDSCs represent a myeloid population that eventually evolve through mature granulocytes [29], we measured the neutrophils count and compared it with lymphocytes count and we found that NLR (neutrophil to lymphocyte ratio) was greater in MM than healthy controls (3.2±0.4% versus 1.6±0.07%, p= 0.0029) and MGUS (1.8±0.08%, p= 0.0002, Figure 1C).
G-MDSC percentage was higher in MM patients carrying NLR ≥2 (46.7±1.9% versus 66.1±1.5%, p< 0.0001, Table 2) and positively correlated to NLR (r2= 0.60, p< 0.0001, Figure 1D).
G-MDSCwb after exposure to novel agents
Then we tested if active drugs in MM, known for their ability to modulate the microenvironment, such as lenalidomide and bortezomib, could affect G-MDSC (Figure 2).
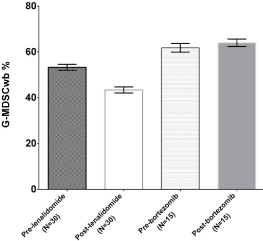
Figure 2: G-MDSC changes after exposure to novel agents.
Changes in percentage of G-MDSC after induction therapy based on
lenalidomide (left bars) or bortezomib (right bars).
After induction therapy (4 Rd or 3 VD/PAD cycles), G-MDSC were reduced after exposure to lenalidomide-based regimen (53.1±1.3 versus 43.4±1.3%, p< 0.0001) but not to bortezomib (61.8±1.9 versus 64.1±1.6%, p= 0.41, Figure 2).
Promising prognostic significance of G-MDSCwb
G-MDSC percentage was not predictor of response or PFS in the whole population (data not shown). However, when we analyzed patients treated upfront with lenalidomide and dexamethasone (N= 30) we found that G-MDSC were associated to poor outcome.
ROC analysis suggested a cut-off of 60% to distinguish patients who maintained response versus who relapsed/progressed, with sensitivity and specificity respectively of 60 and 64%.
All 30 MM patients treated with Rd were evaluable for response after therapy with a median follow up of 28.4 months (range 4.2-69.5 months). All patients except three obtained at least a partial remission (reduction of more than 50% of monoclonal component present at baseline). Patients with G-MDSC ≥60% had a shorter PFS than those with less than 60% (13.9 versus 42.6 months, p= 0.0028, Figure 3A).
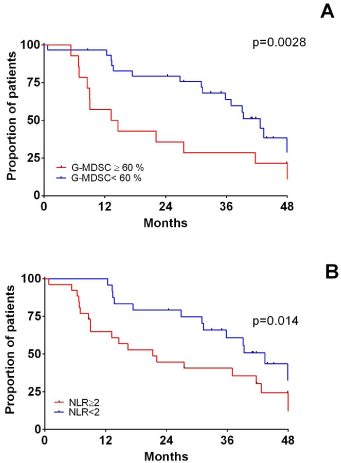
Figure 3: PFS and immune dysregulation in newly diagnosed MM treated
upfront with novel agents.
PFS based on G-MDSCwb at diagnosis (A). PFS in the cohort of patients
treated upfront with lenalidomide is shown based on G-MDSCwb percentage
(B) and NLR (C).
NLR is predictor of PFS
Then, we investigated if NLR , recently described as strong predictors of PFS in MM [30-32] and that we previously found associated to G-MDSC percentage, could have impact on PFS, using cut-off recently published, NLR=2 [30,31].
Considering the whole cohort of patients, NLR was not strong predictors of PFS. However, when we limited our analysis to the subset of patients treated with lenalidomide and dexamethasone, we found that PFS was shorter in patients carrying NLR ≥2 (21.3 versus 43.4 months, p= 0.04, Figure 3B).
Discussion
It is likely that immune suppression is a key element needed in favoring progression from MGUS to MM and we hypothesize that neoplastic plasma cells are able to modify the bone marrow microenvironment, recruiting MDSC in myeloma foci, with inhibition of T-cell activity and consequent immune escape. In this scenario, progression from MGUS to MM is dependent on the progressive reduction of immunological surveillance, that should be monitored during the patient`s follow up and modulated by drugs. However, an affordable method to verify this hypothesis lacks. Information from published data have arisen from mouse models of syngenic tumors [14,15] where MDSC-driven MM expansion was investigated in the background of murine immune system. Moreover, MM model itself appears to be different when developed in mouse or human background [33] and MDSC regulation in mice and humans appear to be really different, even considering the lack of equivalent markers [34].
In this study, we found that MM patients exhibit an increased level of G-MDSC when compared to MGUS and healthy subjects and this population of cells is higher in patients with extensive bone disease, or high LDH or high fibrinogen. We limited our study to peripheral blood to avoid uncertainty of purity in bone-marrow based studies, being the bone marrow invariably contaminated with peripheral blood during aspiration. This is the first large study evaluating the clinical meaning of MDSC in MM patients, since previous reports included 11 newly diagnosed patients [14], 13 relapsed/refractory patients [17], and 11 MGUS [16].
Initially identified in mice for their immunosuppressive function as CD11b+Gr+, human phenotype of MDSC in humans is still controversial [35,36]. We used one of the most accepted phenotypes of MDSC, identified as G-MDSC, previously reported as increased in MM. Previous work confirmed that G-MDSC and mo-MDSC, obtained from frozen mono nucleated fraction of cells in peripheral blood of MM patients are effectively immunosuppressive in in-vitro assay [14,17]. However, recent reports suggested that immunosuppressive properties and antigens in MDSC detection could be modified by freezing-thawing process [36,22]. G-MDSC in particular is extremely cryosensitive [22]. Thus, we evaluated G-MDSC in fresh samples, within two hours from blood collection.
Although present in a small fraction of PBMC, in humans, G-MDSC overlaps for the most part with circulating granulocytes. Arg-1, contained in granulocytes, at the level of azzurrophil granules [23-25], modifies the metabolism of L-arginine, contributes to block translation of the T cell CD3ζ chain and inhibit T cell proliferation. Our previous work (Romano, under revision) showed that neutrophils from MM at diagnosis (MM-N) reduce the expression of activation markers on T-cell surface after exposure to PHA-P, thereby inhibiting capacity of stimulation of T-lymphocytes. The immunosuppressive properties of granulocytes until now have been documented in solid tumors [26], such as in kidney cancer [27] and glioblastoma [23], as well as in hematological neoplasm’s [37-39] but also in healthy subjects [40].
There are emerging experimental observations that in MM there is a large overlapping in immunosuppressive functions of mature granulocytes with G-MDSC, in both humans and mice (Romano, manuscript under revision, [41]), and that the immunosuppressive ability is not limited only to G-MDSC present in the upper Ficolllayer, in the fraction of so-called high-density neutrophils [42,43].
Soluble factors and MM microenvironment can influence G-MDSC expansion. We recently found that MGUS- and MMmesenchymal stem cells are able to induce G-MDSC in vitro from healthy peripheral blood mononuclear cells [44].
Moreover, we found that NLR could be used as G-MDSC surrogates since its positive correlation with G-MDSC percentage in our series. Recent studies involving MM patients treated with either novel agents (VMP, MPT) or older schemes (MP, VAD) suggested that NLR >2 at diagnosis is predictors of PFS and OS [30-32]. We speculated that the prognostic meaning of NLR was due to its ability to represent the myeloid-driven immunological impairment.
G-MDSC seems to be clinically relevant in patients treated upfront with lenalidomide. Our data suggest that high levels of G-MDSC >60% are associated with poor outcome and, on the other hand, lenalidomide can induce MDSC reduction. However, we need deeper details to address this question. Although, in vitro studies exclude a direct effect of lenalidomide or bortezomib in MDSC expansion [17], it has been shown that lenalidomide can reduce cytokines involved in MDSC recruitment [17]. In addition, in lymphoma-bearing mice, lenalidomide can reduce MDSC numbers and reverting cancer-induced immunosuppression [45]. Little is known about the direct effect of novel agents on micro-environment, thus more mechanicistic experiments are ongoing in our laboratory to explain the biological meaning of our clinical observations.
In conclusion, we reported how the myeloid immunological impairment driven by G-MDSC and related granulocytes could impact on clinical outcome in MM. We found that MM, more than MGUS, patients exhibit an increased levels of circulating G-MDSC in peripheral blood. We speculated that MM expansion needs immunoparesis in order to maintain poor tumor-specific immune response. In this perspective, cells belonging to myeloid compartment, both as immature (G-MDSC) or more mature cells (neutrophils) have the ability to suppress T lymphocytes activation and this phenomenon is reflected by the neutrophil to lymphocyte ratio (NLR), a parameter that can be easily evaluated in every single patient and can provide prognostic information. In addition, the amount of these myeloid cells can be modulated by lenalidomide but not by bortezomib, adding further information on the complex mechanism of action of immunomodulating drugs.
Acknowledgment
This work has been supported in part by Associazione Italiana contro le Leucemie (AIL) of Catania, Fondazione Catanese per lo Studio e la Cura delle Malattie Neoplastiche del Sangue (FON.CA.NE. SA), and Ministero della Salute, Ricerca Finalizzata 2011-2012 (PE- 2011-02350147).
References
- Harousseau JL1 . Ten years of improvement in the management of multiple myeloma: 2000-2010. See comment in PubMed Commons below Clin Lymphoma Myeloma Leuk. 2010; 10: 424-442.
- Kyle RA1, Steensma DP . History of multiple myeloma. See comment in PubMed Commons below Recent Results Cancer Res. 2011; 183: 3-23.
- Kyle RA1, Rajkumar SV . Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. See comment in PubMed Commons below Curr Hematol Malig Rep. 2010; 5: 62-69.
- Davies FE1, Dring AM, Li C, Rawstron AC, Shammas MA, O'Connor SM, Fenton JA . Insights into the multistep transformation of MGUS to myeloma using microarray expression analysis. See comment in PubMed Commons below Blood. 2003; 102: 4504-4511.
- Landgren O1, Korde N . Multiple myeloma precursor disease: current clinical and epidemiological insights and future opportunities. See comment in PubMed Commons below Oncology (Williston Park). 2011; 25: 589-590.
- Zingone A1, Kuehl WM . Pathogenesis of monoclonal gammopathy of undetermined significance and progression to multiple myeloma. See comment in PubMed Commons below Semin Hematol. 2011; 48: 4-12.
- Brousseau M, Leleu X, Gerard J, Gastinne T, Godon A, Genevieve F, et al. Hyperdiploidy is a common finding in monoclonal gammopathy of undetermined significance and monosomy 13 is restricted to these hyperdiploid patients. Clin Cancer Res. 2007; 13: 6026-6031.
- Kaufmann H, Ackermann J, Baldia C, Nösslinger T, Wieser R, Seidl S, et al. Both IGH translocations and chromosome 13q deletions are early events in monoclonal gammopathy of undetermined significance and do not evolve during transition to multiple myeloma. Leukemia. 2004; 18: 1879-1882.
- López-Corral L, Gutiérrez NC, Vidriales MB, Mateos MV, Rasillo A, García-Sanz R, et al. The progression from MGUS to smoldering myeloma and eventually to multiple myeloma involves a clonal expansion of genetically abnormal plasma cells. Clin Cancer Res. 2011; 17: 1692-1700.
- Dhodapkar MV1, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, Krasovsky J . A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. See comment in PubMed Commons below J Exp Med. 2003; 197: 1667-1676.
- 11 Pratt G1, Goodyear O, Moss P . Immunodeficiency and immunotherapy in multiple myeloma. See comment in PubMed Commons below Br J Haematol. 2007; 138: 563-579.
- Racanelli V1, Leone P, Frassanito MA, Brunetti C, Perosa F, Ferrone S, Dammacco F . Alterations in the antigen processing-presenting machinery of transformed plasma cells are associated with reduced recognition by CD8+ T cells and characterize the progression of MGUS to multiple myeloma. See comment in PubMed Commons below Blood. 2010; 115: 1185-1193.
- Freeman LM, Lam A, Petcu E, Smith, R., Salajegheh, A, Diamond P, et al. Myeloma-induced alloreactive T cells arising in myeloma-infiltrated bones include double-positive CD8+CD4+ T cells: evidence from myeloma-bearing mouse model. J Immunol. 2011; 187: 3987-3996.
- Ramachandran IR1, Martner A, Pisklakova A, Condamine T, Chase T, Vogl T, Roth J . Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. See comment in PubMed Commons below J Immunol. 2013; 190: 3815-3823.
- Van Valckenborgh E, Schouppe E, Movahedi K, De Bruyne E, Menu E, De Baetselier P, Vanderkerken K . Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. See comment in PubMed Commons below Leukemia. 2012; 26: 2424-2428.
- Brimnes MK1, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, Svane IM . Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DRâ»/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. See comment in PubMed Commons below Scand J Immunol. 2010; 72: 540-547.
- Görgün GT1, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, Raje N . Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. See comment in PubMed Commons below Blood. 2013; 121: 2975-2987.
- Nagaraj S1, Collazo M, Corzo CA, Youn JI, Ortiz M, Quiceno D, Gabrilovich DI . Regulatory myeloid suppressor cells in health and disease. See comment in PubMed Commons below Cancer Res. 2009; 69: 7503-7506.
- Srivastava MK1, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S . Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. See comment in PubMed Commons below Cancer Res. 2010; 70: 68-77.
- Peranzoni E1, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V . Myeloid-derived suppressor cell heterogeneity and subset definition. See comment in PubMed Commons below Curr Opin Immunol. 2010; 22: 238-244.
- Romano A1, Vetro C, Adriani M . Advances in understanding regulatory myeloid cells. See comment in PubMed Commons below Cancer Biol Ther. 2011; 11: 923-926.
- Durie BG1, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, Gertz M . International uniform response criteria for multiple myeloma. See comment in PubMed Commons below Leukemia. 2006; 20: 1467-1473.
- Sica A1, Porta C, Morlacchi S, Banfi S, Strauss L, Rimoldi M, Totaro MG . Origin and Functions of Tumor-Associated Myeloid Cells (TAMCs). See comment in PubMed Commons below Cancer Microenviron. 2012; 5: 133-149.
- Kelkitli E1, Atay H, Cilingir F, Güler N, Terzi Y, Ozatlı D, Turgut M . Predicting survival for multiple myeloma patients using baseline neutrophil/lymphocyte ratio. See comment in PubMed Commons below Ann Hematol. 2014; 93: 841-846.
- Shin SJ1, Roh J1, Kim M1, Jung MJ1, Koh YW1, Park CS1, Yoon DH2 . Prognostic significance of absolute lymphocyte count/absolute monocyte count ratio at diagnosis in patients with multiple myeloma. See comment in PubMed Commons below Korean J Pathol. 2013; 47: 526-533.
- Romano A1,2, Parrinello NL3, Consoli ML3, Marchionni L4, Forte S5, Conticello C3 . Neutrophil to lymphocyte ratio (NLR) improves the risk assessment of ISS staging in newly diagnosed MM patients treated upfront with novel agents. See comment in PubMed Commons below Ann Hematol. 2015; 94: 1875-1883.
- Tassone P1, Neri P, Carrasco DR, Burger R, Goldmacher VS, Fram R, Munshi V . A clinically relevant SCID-hu in vivo model of human multiple myeloma. See comment in PubMed Commons below Blood. 2005; 106: 713-716.
- Talmadge JE1, Gabrilovich DI . History of myeloid-derived suppressor cells. See comment in PubMed Commons below Nat Rev Cancer. 2013; 13: 739-752.
- Gabrilovich DI1, Ostrand-Rosenberg S, Bronte V . Coordinated regulation of myeloid cells by tumours. See comment in PubMed Commons below Nat Rev Immunol. 2012; 12: 253-268.
- Trellakis S1, Bruderek K, Hütte J, Elian M, Hoffmann TK, Lang S, Brandau S . Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer. See comment in PubMed Commons below Innate Immun. 2013; 19: 328-336.
- Kotsakis A1, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL . Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. See comment in PubMed Commons below J Immunol Methods. 2012; 381: 14-22.
- Sippel TR1, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, Waziri A . Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. See comment in PubMed Commons below Clin Cancer Res. 2011; 17: 6992-7002.
- Munder M1, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes JM, Luckner C . Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. See comment in PubMed Commons below Blood. 2005; 105: 2549-2556.
- Munder M1, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, Kropf P . Suppression of T-cell functions by human granulocyte arginase. See comment in PubMed Commons below Blood. 2006; 108: 1627-1634.
- Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012; 61: 1155-1167.
- Rodriguez PC1, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC . Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. See comment in PubMed Commons below Cancer Res. 2009; 69: 1553-1560.
- Giallongo C, Parrinello N, Tibullo D, La Cava P, Romano A, Chiarenza A. Myeloid derived suppressor cells (MDSCs) are increased and exert immunosuppressive activity together with polymorphonuclear leukocytes (PMNs) in chronic myeloid leukemia patients. PLoS One. 2014; 9: e101848.
- Romano A1, Conticello C2, Cavalli M2, Vetro C3, La Fauci A2, Parrinello NL3, Di Raimondo F3 . Immunological dysregulation in multiple myeloma microenvironment. See comment in PubMed Commons below Biomed Res Int. 2014; 2014: 198539.
- Romano A1, Parrinello NL, Vetro C, Forte S, Chiarenza A, Figuera A, Motta G . Circulating myeloid-derived suppressor cells correlate with clinical outcome in Hodgkin Lymphoma patients treated up-front with a risk-adapted strategy. See comment in PubMed Commons below Br J Haematol. 2015; 168: 689-700.
- Munder M1 . Arginase: an emerging key player in the mammalian immune system. See comment in PubMed Commons below Br J Pharmacol. 2009; 158: 638-651.
- Ramachandran IR1, Condamine T2, Lin C3, Herlihy SE3, Garfall A4, Vogl DT4, Gabrilovich DI2 . Bone marrow PMN-MDSCs and neutrophils are functionally similar in protection of multiple myeloma from chemotherapy. See comment in PubMed Commons below Cancer Lett. 2016; 371: 117-124.
- Pillay J1, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH . A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. See comment in PubMed Commons below J Clin Invest. 2012; 122: 327-336.
- Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013; 70: 3813-3827.
- Giallongo C, Parrinello N, Tibullo D, P. La Cava, C. Conticello, F. Puglisi, A. Granulocyte-like Myeloid Derived Suppressor Cells (G-MDSCs) are increased in Multiple Myeloma due to immunological dysregulation of mesenchymal stem cells (MSCs). Clinical Lymphoma Myeloma and Leukemia. 15: e211.
- Sakamaki I1, Kwak LW1, Cha SC1, Yi Q1, Lerman B1, Chen J2, Surapaneni S2 . Lenalidomide enhances the protective effect of a therapeutic vaccine and reverses immune suppression in mice bearing established lymphomas. See comment in PubMed Commons below Leukemia. 2014; 28: 329-337.