
Case Report
Ann Hematol Oncol. 2016; 3(2): 1079.
Two Cases with CD-20 Negative Diffuse Large B-Cell Lymphoma and Literature Review
Miranda-Aquino T*, Pérez-Topete SE and Montemayor-Montoya JL
Department of Internal Medicine, Hospital Christus Muguerza, Mexico
*Corresponding author: Tomas Miranda Aquino, Department of Internal Medicine, Hospital Christus Muguerza, UDEM, 1ra Av 758 Jardines de Anáhuac, San Nicolás de los Garza, Nuevo León, México
Received: March 24, 2016; Accepted: May 18, 2016; Published: May 20, 2016
Abstract
CD 20 negative diffuse large B-cell lymphoma is a very rare and aggressive neoplasm. We report two subtypes of this neoplasm: anaplastic lymphoma kinase positive diffuse large B-cell lymphoma and a plasmablastic lymphoma. These pathologies are very difficult to diagnose and treat. The first case was anaplastic lymphoma kinase positive diffuse large B-cell lymphoma, the patient received multiple schemes of chemotherapy with a torpid evolution and the patient died. The second case is a plasmablastic lymphoma, the patient was treated with infusional etoposide, vincristine, doxorubicin, cyclophosphamide and prednisone dose-adjusted chemotherapy, the treatment was successful with complete remission and without relapses.
Keywords: CD 20 negative diffuses large B-cell lymphoma; Diffuse large B-cell lymphoma ALK positive; Plasmablastic lymphoma; Infusional DAEPOCH; CHOP-Bleo
Abbreviations
ALK+DLBCL: Anaplastic Lymphoma Kinase Positive, Diffuse Large B-cell Lymphoma; PBL: Plasmablastic Lymphoma; CD20-: CD20 Negative; DLBCL: Diffuse Large B-cell Lymphoma; CHOPBleo: Cyclophosphamide, Daunorubicin, Vincristine, Prednisone and Bleomycin; IPI: International Prognostic Index; ICE: Ifosfamide, Carboplatin and Etoposide; PET: Positron Emission Tomography; CT: Computer Tomography; PET-CT: Positron Emission Tomography- Computed Tomography ; DA-EPOCH: Dose-adjusted Etoposide, Vincristine, Doxorubicin, Cyclophosphamide and Prednisone; LDH: Lactate Dehydrogenase; HIV: Human Immunodeficiency Virus; ALK: Anaplastic Lymphoma Kinase; CTLC-ALK; ALCL: Anaplastic Large cell Lymphoma; CD30; CD45; EMA: Epithelial Membrane Antigen; CD20; CD79a; PAX-5; CD138; CD4; MUM-1; IRF4; PFS: Progression-Free Survival; OS: Overall Survival; NCCN: National Comprehensive Cancer Network; Hyper CVAD: Hyperfractionated Cyclophosphamide, Vincristine, Doxorubicin and Dexamethasone alternating with cytarabine and methotrexate; CODOX-M/ IVAC: High dose Methrotrexate/Ifosfamide, Etoposide, and High dose Cytarabine; CHOP: Cyclophosphamide, Daunorubicin, Vincristine, Prednisone; GC: Non-germinal Center; SCT: Stem Cell Transplant; IMVP: Ifosfamide, Methotrexate and Etoposide; DHAP: Dexamethasone, high-dose Cytabine, cisplatin; PBSCT: Peripheral Blood Stem Cell Transplantation; PVDA: Prednisone, Vincristine, Doxorubicin and Asparaginase; R-CHOP: Rituximab- Cyclophosphamide, Daunorubicin, Vincristine, Prednisone; LNH96- 2002; BFM-90; LMB-89; LN: Lymph Node; NED: No Evidence of Disease
Introduction
Anaplastic lymphoma kinase positive, diffuse large B-cell lymphoma (ALK+DLBCL) and plasmablastic lymphoma (PBL) are two very rare subtypes of diffuse large B-cell lymphoma (DLBCL). They are aggressive neoplasm of B cells [1], CD20 negative (CD20-) and with a high proliferation index [2], with poor response to current therapies [3]. We report two cases with this diagnosis.
Case 1
A 44-year-old man without past medical history was diagnosed with ALK+DLBCL with plasmacytoid differentiation (Figure 1A,1B) in the context of supraclavicular lymphadenopathy and B symptoms. An abdominal computer tomography (Figure 2) revealed splenomegaly and retroperitoneal lymphadenopathy. The bone marrow biopsy was negative for malignant cell infiltration; clinical stage IIIA, and an international prognostic index (IPI) of 3 points. Six cycles of cyclophosphamide, daunorubicin, vincristine, prednisone and bleomycin (CHOP-Bleo) chemotherapy was indicated, but without improvement. A second line chemotherapy consisting of three cycles of ifosfamide, carboplatin and etoposide (ICE) was administered. At the final of the chemotherapy the patient had symptomatic improvement but a positron emission tomography (PET) scan showed lymphoma activity. A stem cell collection for autologous stem cell transplant (SCT) was unsuccessful; therefore, 25 sessions of retroperitoneal and spleen radiotherapy were given.
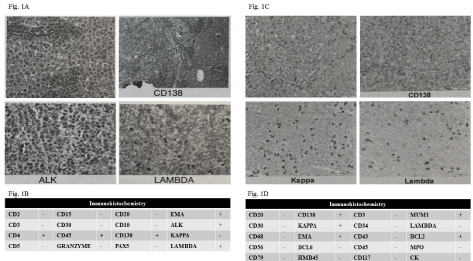
Figure 1: Figure 1A: Histopathology of a diffuse large B-cell ALK positive lymphoma.
Figure 1B: Immunohistochemistry of the diffuse large B-cell ALK positive lymphoma.
Figure 1C: Histopathology of a plasmablastic lymphoma.
Figure 1D: Immunohistochemistry a plasmablastic lymphoma.
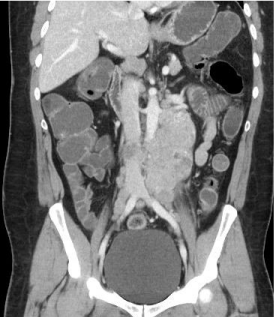
Figure 2: Abdominal computed tomography with an important retroperitoneal
lymphadenopathy.
The patient was admitted to the hospital with diffuse abdominal pain. During his hospitalization new cycle with infusional doseadjusted etoposide, vincristine, doxorubicin, cyclophosphamide and prednisone (DA-EPOCH) and bortezomib scheme was received.
He developed secondary pancytopenia which was treated with transfusions and filgrastim. Painful hepatomegaly and persistent highly elevated lactate dehydrogenase (LDH) were suggestive of tumor activity.
A new computer tomography (CT) (Figure 3) showed no changes in tumoral activity compared with the pre-EPOCH images. He persisted with clinical deterioration, with torpid evolution and died with progressive tumoral progression.
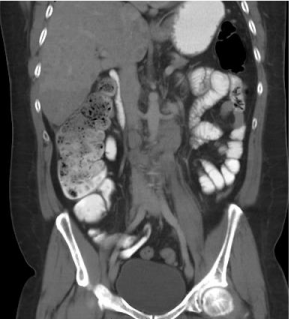
Figure 3: Abdominal computed tomography with the presence of remanent
retroperitoneal lymphadenopathy.
Case 2
A 77-year-old man with history of arterial hypertension and ischemic heart disease, HIV negative. A diagnosis of plasmablastic lymphoma (PBL) had been made when the patient presented mucous stools and colonoscopy was performed showing 5 polyps in colon (Figure 4) that were resected and biopsied. Pathological examination revealed PBL (Figure 1C,1D). Clinical stage IIIB, and an IPI of 3 points.
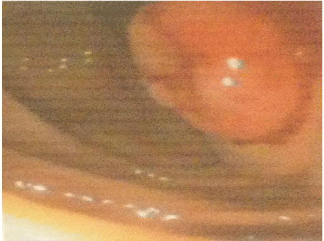
Figure 4: A colonoscopy with the presence of a polyp in the sigmoid colon.
PET-CT scan presented hyper metabolic activity of cervical, mediastinal and retroperitoneal lymphadenopathy. Aspiration and bone marrow biopsy were negative for lymphoma infiltration. Serum protein immune electrophoresis exhibited high levels of IgG (2525 mg/dl). Normal Inmunofixation pattern was observed.
Six cycles of infusional DA-EPOCH chemotherapy was administered. The patient presented neuropathy as side effect without pancytopenia and LDH levels were normal. Ten months posteriorly of the diagnosis, follow up colonoscopy showed no evidence of lymphoma activity and a PET-CT scan was normal.
Discussion
DLBCL is an aggressive neoplasm of B cells, which accounts for almost 40% of all non- Hodgkin lymphoma cases. It is a disease marked by heterogeneity in clinical presentation, morphology, and underlying biology. As such, patient outcome is variable; 5-year survival is approximately 50% [1].
CD20- DLBCL is very rare. The vast majority of reported cases mainly occur in a few variant subtypes of DLBCL, including PBL and ALK+DLBCL; however, data on CD20- de novo DLBCL patients are largely limited to HIV-infected or other immune compromised patients [2].
CD20-DLBCL is more closely associated with aggressive pathologic parameters than CD20 positive DLBCL, with a higher proliferation index [3]. The presence of immunoblastic and occasionally plasmablastic tumor cells coupled with expression of plasma cell markers and lack of expression of B-cell-associated markers is typical for ALK+DLBCL and plasmablastic lymphoma [4].
ALK+DLBCL is characterized by plasmablastic morphologic features and a translocation involving the ALK gene, most often t (2;17) (p23;q23), leading to a CLTC-ALK fusion product. Tumor cells of ALK+DLBCL do not express markers of B-cell lineage such as CD20 and CD79a. They do, however, express markers of terminally differentiated B cells/plasma cells, includingVersus38, CD138, and MUM-1. They are also typically positive for epithelial membrane antigen (EMA) and usually aberrantly express the T-cell marker CD4. The immunohistochemical expression of the ALK protein is cytoplasmic and granular, usually with Golgi staining as well [5].
ALK+DLBCL have several clinic pathological differences from ALK-positive anaplastic large cell lymphoma (ALCL) [6]. Importantly, ALK+DLBCL does not express CD30 [7,8].
Since the original description of this hematological entity, multiple cases of ALK+DLBCL have been reported in the last 10 years (Table 1), mostly described as small case series or case reports. The largest case series described was done by Laurent et al. [9] and consisted of a retrospective analysis of 38 cases of ALK+DLBCL with detailed clinical and pathologic descriptions of the tumors. In this series, most of the patients were older than 40 years; with a 5:1 male/female ratio; 60% had advanced disease at diagnosis (stage III/IV); mainly with diffuse lymph node infiltration and with apparently high but not reported IPI score; had aggressive evolution with poor response to cyclophosphamide, daunorubicin, vincristine, prednisone (CHOP) or CHOP-like regimens and approximately half of them died within the first 12 months of diagnosis [4].
Reference
Case
Age
Sex
Primary site
Therapy
Survival
Outcome
[16]
1
26
F
Axillary LN
CHOP, DHAP
6 m
Lost follow-up
2
35
M
Axillary LN
CHOP, IMVP, DHAP, ICE, Radiation
18 m
Died
3
24
M
Cervical LN
CHOP, IMVP, PBSCT, PVDA, DHAP, Radiation
17 m
Died
[5]
1
27
M
Bone
Hyper-CVAD
11 m
Alive, with disease
2
41
F
Nasal fossa
Radiotherapy
13 m
Alive, NED
3
13
F
Cervical LN
LNH96-2002
62 m
Alive, NED
4
70
M
Cervical LN
CHOP
72 m
Alive, NED
[17]
1
32
M
Cervical LN
CHOP, Radiation
50 m
Alive, NED
[4]
1
39
M
Cervical LN
R-CHOP
2 m
Died
[18]
1
26
M
Duodenum
EPOCH
ND
Alive, with disease
[19]
1
9
M
LN
BFM-90, LMB-89, Radiation
5 m
Died
This report
1
44
M
Cervical LN
CHOP-bleo, ICE, Radiotherapy, DA EPOCH, Bortezomib
15 m
Died
LN: Lymph Node; NED: No Evidence of Disease; CHOP: Cyclophosphamide, Doxorubicin, Vincristine, Prednisone; DHAP: Dexamethasone, Cytarabine and Cisplatinum; IMVP: Ifosfamide, Methotrexate and Etoposide; ICE: Ifosfamide, Carboplatin and Etoposide; PBSCT: Peripheral Blood Stem Cell Transplantation; PVDA: Prednisone, Vincristine, Doxorubicin and Asparaginase; Hyper-CVAD: Hyperfractionated Cyclophosphamide, Vincristine, Doxorubicin and Dexamethasone Alternating with Cytarabine and Methotrexate; EPOCH: Etoposide, Vincristine, Doxorubicin, Cyclophosphamide and Prednisone; DA EPOCH: Dose-Adjusted Etoposide, Vincristine, Doxorubicin, Cyclophosphamide and Prednisone.
Table 1: Clinical features of literature patients with AKL+ DLBCL.
ALK-positive large B-cell lymphoma is an aggressive lymphoid neoplasm, fatal in 56% of the cases. The most common cause of death the progression of the lymphoma, observed in 90% of the reported cases [7].
PBL was first described in 1997 by Delecluse et al. [10]. In this report, 16 patients, of whom 15 were infected with human immunodeficiency virus (HIV), presented with an oral mass and aggressive clinical behavior, poor response to conventional therapies, and short survival. PBL is now recognized by the World Health Organization as one of the lymphomas that is more commonly seen in patients with HIV infection [8].
PBL remains a diagnostic challenge given its peculiar morphology and an immunohistochemical profile similar to plasma cell myeloma. Pathologically, PBL is characterized by a monomorphic proliferation of large, round to oval-shaped cells with abundant cytoplasm, eccentric nucleus, and prominent nucleolus. The background is composed of lymphocytes, apoptotic and mitotic figures and macrophages that impart a “starry-sky appearance”. PBL cells show a plasmacytic immunophenotype, in which classic B cell markers, such as CD20, CD45, CD79a, and PAX-5, are lost. However, plasma cell markers, such as CD38, CD138, and MUM-1/IRF4, are expressed [5].
PBL has been characterized for its predilection of involving the oral cavity of HIV-positive individuals. However, extra oral involvement is also frequently seen in HIV-positive cases; the most commonly affected sites are the gastrointestinal tract, lymph nodes, and skin. A similar pattern is seen in patients with HIV-negative PBL, with the oral cavity and gastrointestinal tract being the most commonly involved sites [11,12].
The diagnosis of PBL is clinically relevant, as this type of mature B cell neoplasm, acts aggressively, responds poorly to therapy and has a lower progression-free survival (PFS) and overall survival (OS) than other types of DLBCL. Clear-cut differences from conventional DLBCL were found in response rates and survival. Even when compared solely with non-germinal center (GC) type DLBCL, the most aggressive subtype of DLBCL, the differences in OS and PFS with PBL were significant [13].
Recently, Castillo et al. [14] described a systematic review of 76 HIV-negative PBL patients and showed a median OS of 9 months with a 2-year OS rate of 10%. Actually, there is no standard of care for patients with PBL, therefore the prognosis is poor. In particular, the use of CHOP is considered inadequate therapy, and current guidelines recommend more intensive regimens. Castillo et al. [14] recommend for first-line treatment of PBL, 6 cycles of infusional DAEPOCH (with or without bortezomib) plus intrathecal prophylaxis with each cycle of EPOCH and consideration of consolidative high dose chemotherapy followed by autologous SCT in first remission for appropriate candidates.
A lack of active targeted therapy for CD20- DLBCL, like ALK+DLBCL and PBL, might also contribute to the poor prognosis [4,5]. It is reported that CHOP-Bleo regimens have been used in aggressive DLBCL [7]. The National Comprehensive Cancer Network (NCCN) guidelines recommend against CHOP in favor of more intensive regimens, such as EPOCH, hyper fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternating with cytarabine and methotrexate (Hyper CVAD), or cyclophosphamide, vincristine, doxorubicin, high dose methrotrexate/ifosfamide, etoposide, and high dose cytarabine (CODOX-M/IVAC) [8-10]. There are few case reports on the use of bortezomib [7].
Tyrosine kinase receptor becomes more relevant in the sense that there are currently clinical trials evaluating the anti tumoral activity of ALK inhibitors. Kwak, et al. [15] evaluated the activity of crizotininb, an orally available small molecule inhibitor of ALK, in patient with ALK+ non-small cell lung cancer and showed positive biological activity of tumor shrinkage and stable disease in most patients with only mild grade gastrointestinal adverse effects.
Despite the advances, these two diseases are difficult to diagnose, both share aggressive clinical curse, refractory to treatment and high mortality, even with the novel therapeutics.
Case 1 died despite having received multiple chemotherapy regimens. They were indicated despite little evidence in the literature of management of this condition. In case 2, the patient improved, with complete remission of the disease without relapse to date.
In conclusion DLBCL CD20- show very aggressive behavior, high relapse rate and lack of response to standard regimens.
References
- Wilkinson TS, Vanpatten KA, Fernandez DR, Brunhoeber P, Garsha KE, Glinsmann-Gibson BJ, et al. Partial plasma cell differentiation as a mechanism of lost major histocompatibility complex class II expression in diffuse large B-cell lymphoma. Blood. 2012; 119: 1459-1467.
- Li YJ, Li ZM, Rao HL, Xia Y, Huang HQ, Xia ZJ, et al. CD20-negative de novo diffuse large B-cell lymphoma in HIV-negative patients: A matched case-control analysis in a single institution. J Transl Med. 2012; 10: 84.
- Montes-Moreno S, Gonzalez-Medina AR, Rodríguez Pinilla SM, Maestre L, Sánchez-Velarde L, Roncador G, et al. Aggressive large B-cell lymphoma with plasma cell differentiation: immunohistochemical characterization of plasmablastic lymphoma and diffuse large B-cell lymphoma with partial plasmablastic phenotype. Haematologica. 2010; 95:1342-1349.
- Chapman-Fredricks J, Blieden C, Sandoval JD, Ernani V, Ikpatt OF. Acute Spontaneous Tumor Lysis Syndrome as the Initial Presentation of ALK-Positive Diffuse Large B-Cell Lymphoma. Appl Immunohistochem Mol Morphol. 2014; 22: 317–321.
- Beltran B, Castillo J, Salas R, Quiñones P, Morales D, Hurtado F, et al. AKL-positive diffuse large B.cell lymphoma: report of four cases and review of the literature. J Hematol Oncol. 2009; 2: 11.
- Valera A, Colomo L, Martínez A, de Jong D, Balagué O, Matheu G, et al. ALK-positive large B-cell lymphomas express a terminal B-cell differentiation program and activated STAT3 but lack MYC rearrangements. Mod Pathol. 2013; 26: 1329-1337.
- Avilés A, Cleto S, Huerta-Guzmán J, Neri N. Interferon alfa 2b as maintenance therapy in poor risk diffuse large B-cell lymphoma in complete remission after intensive CHOP-BLEO regimens. Eur J Haematol. 2001; 66: 94-99.
- Castillo JJ, Reagan JL. Plasmablastic Lymphoma: A systematic Review. Scientific World Journal. 2011; 11: 687-696.
- Laurent C, Do C, Gascoyen RD,Gascoyne RD, Lamant L, Ysebaert L, et al. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma: a rare clinicopathologic entity with poor prognosis. J Clin Oncol. 2009; 27: 4211–4216.
- Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997; 89: 1413-1420.
- Hapgood G, Savage K. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015; 126: 17-25.
- Al-MAlki MM, Castillo JJ, Sloan JM, Re A. Hematopoietic Cell Transplantation for Plasmablastic Lymphoma: A review. Biol Blood Marrow Transplant. 2014; 20: 1877-1884.
- Jacobsen ED, Sharman JP, Oki Y, Advani R, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015; 125: 1394-1402.
- Castillo J, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. BLOOD. 2015; 125: 2323-2330.
- Kwak E, Bang Y, Camidge R, Shaw A, Solomon B, Maki RG, et al. Anaplastic Lymphoma Kinase Inhibition in Non–Small-Cell Lung Cancer. N Engl J Med. 2010; 363: 1693-1703.
- Lee HW, Kim K, Kim W, Ko YH. ALK-positive diffuse large B-cell lymphoma: report of three cases. Hematol Oncol. 2008; 26: 108-113.
- Zhang D, Denley R, Filippa D, Teruya-Feldstein J. ALK-positive Diffuse Large B-cell Lymphoma With the t(2;17) (p23;q23). Appl Immunohistochem Mol Morphol. 2009; 17: 172-177.
- Xing X, Lin D, Ran W, Liu H. ALK-positive diffuse large B-cell lymphoma of the duodenum: A case report and review of the literature. Experimental and Therapeutic Medicine. 2014; 8: 409-412.
- Bubala H, Maldyk J, Wlodarska I, Sonta-Jakimczyk D, Szczepanski T. ALK-Positive Diffuse Large B-Cell Lymphoma. Pediatr Blood Cancer. 2006; 46: 649-653.