
Case Report
Ann Hematol Oncol. 2016; 3(3): 1080.
Nonbacterial Thrombotic Endocarditis of the Tricuspid Valve Associated with Pancreatic Adenocarcinoma: A Case Report
Sochat MM¹*, Kailasam MT² and Dotan E³
¹Department of Internal Medicine, Temple University Hospital, USA
²Department of Internal Medicine, Fox Chase Cancer Center, USA
³Department of Medical Oncology, Fox Chase Cancer Center, USA
*Corresponding author: Matthew M Sochat, Department of Internal Medicine, Temple University Hospital, 3401 North Broad Street, Philadelphia, USA
Received: March 31, 2016; Accepted: May 25, 2016; Published: May 27, 2016
Abstract
Nonbacterial thrombotic endocarditis, also known as marantic endocarditis, is a disorder characterized by the deposition of sterile vegetations on the heart valves. It occurs in the context of noninfectious chronic inflammatory conditions, particularly visceral malignancies. These vegetations are much more prone to detach and embolize as compared to infectious endocarditis, and patients typically present with the signs and symptoms of systemic arterial emboli, such as ischemic cerebral infarctions and acute coronary syndromes. The left heart valves are most commonly involved, although right heart valve involvement can rarely be seen. It is typically managed by treating the underlying cause of inflammation and administering anticoagulation. Here we present the case and associated literature review of a patient with metastatic pancreatic tail adenocarcinoma, prior deep venous thromboses and pulmonary emboli on rivaroxaban therapy, presenting with ischemic strokes secondary to nonbacterial thrombotic endocarditis, with tricuspid valve involvement.
Keywords: Anticoagulation; Endocarditis; Nonbacterial; Pancreatic neoplasms; Thrombosis; Tricuspid valve
Abbreviations
CT: Computed Tomography; DCIS: Ductal Carcinoma In Situ; DIC: Disseminated Intravascular Coagulation; DVT: Deep Venous Thrombosis; DWI: Diffusion-Weighted Imaging; MRI: Magnetic Resonance Imaging; NBTE: Nonbacterial Thrombotic Endocarditis; NOAC: Novel Oral Anticoagulant; PE: Pulmonary Embolism; SLE: Systemic Lupus Erythematosus; TEE: Transesophageal Echocardiography; TTE: Transthoracic Echocardiography; VTE: Venous Thromboembolism
Case Presentation
A 74-year-old female presented to our urgent care center with one day of generalized weakness, nausea, vomiting, gait imbalance, and numerous falls. She reported no similar symptoms prior to this and denied any focal symptoms including weakness or sensory loss. She denied both systemic symptoms of fever, chills and sweats as well as focal respiratory, urinary or gastrointestinal symptoms; her appetite was only modestly decreased. One month prior to this presentation, she was diagnosed with adenocarcinoma of the pancreatic tail with metastatic disease to the lungs and liver. This was confirmed with a biopsy of a metastatic liver lesion. Simultaneously she was found to have bilateral deep venous thromboses (DVTs) and pulmonary emboli (PEs). She had been initiated on rivaroxaban therapy at that time. One week prior, she had presented to our institution for consideration of systemic chemotherapy.
During her initial evaluation, her vital signs were stable, and she was afebrile. Orthostatic vital signs were negative. Initial neurological exam was notable for gait ataxia, but was otherwise non-focal. Cardiopulmonary examination revealed clear lungs and no evidence of abnormal heart sounds. She had stable right upper extremity lymphedema following a remote lumpectomy 28 years prior for ductal carcinoma in situ (DCIS), and no evidence of lower extremity edema. The abdomen was mildly tender throughout but otherwise benign, the remainder of her initial examination was normal.
An initial complete blood count revealed hemoglobin of 13.1 g/ dL, white blood cell count of 15,000 cells/mm3 and platelet count of 192,000/mm3. Her comprehensive metabolic panel showed alkaline phosphatase of 218 units/L, but was otherwise unremarkable. Noncontrast computed tomography (CT) scan of the head was performed and negative for any pathology. She was admitted for observation and rehydration overnight.
Serial neurological exams over the course of the following day revealed the evolution of left hemineglect and subsequently left face, arm, and leg weakness and sensory deficits that were not seen on admission. Magnetic resonance imaging (MRI) of the brain was performed, which revealed scattered acute bilateral anterior and posterior circulation ischemic infarctions on diffusion weighted imaging (DWI) sequences (Figure 1). A subsequent bilateral carotid artery duplex study was unremarkable. Transthoracic echocardiography (TTE) revealed a medium-sized (11 mm by 8 mm), verrucoid, solid, fixed vegetation on the tip of the posterior tricuspid valve leaflet (Figures 2, 3). No left-sided vegetations were detected and all other cardiac parameters, including tricuspid valve function, were normal, although a bubble study was not performed, and it is not known if an intra-cardiac shunt was present.

Figure 1: DWI sequences on brain MRI reveal numerous bilateral acute
ischemic infarctions in both the anterior and posterior circulations.
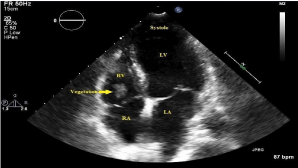
Figure 2: TTE demonstrating a medium-sized (11mm by 8mm), verrucoid,
solid, fixed vegetation on the tip of the posterior tricuspid valve leaflet, during
systole.

Figure 3: TTE demonstrating a medium-sized (11mm by 8mm), verrucoid,
solid, fixed vegetation on the tip of the posterior tricuspid valve leaflet, during
diastole.
She remained afebrile and her leukocytosis improved; blood and urine cultures were negative. Given her known hypercoagulable state and failure to meet the modified Duke criteria, infectious endocarditis was deemed unlikely and a diagnosis of NBTE was strongly considered, presumptively with bilateral heart involvement despite confirmation of involvement only within the right heart. A bubble study during TTE was not performed. With input from the neurology service, the patient’s therapeutic anticoagulation was transitioned from rivaroxaban to enoxaparin. Following this change, no further neurological deficits developed, and the patient had fair performance on both physical therapy assessments and speech/ swallow testing. Unfortunately, her neurological deficits did not improve, and her performance status precluded further treatment of her advanced pancreatic malignancy. She was ultimately discharged home with hospice services.
Discussion
This case report describes a patient with a recently diagnosed metastatic pancreatic adenocarcinoma presenting with evolving ischemic cerebral infarctions secondary to suspected nonbacterial thrombotic endocarditis (NBTE). NBTE is an uncommon hypercoagulable condition, which results in the development of sterile vegetations on the heart valves. This noninfectious pathology is most commonly associated with advanced visceral malignancies and systemic inflammatory processes such as systemic lupus erythematosus (SLE) and disseminated intravascular coagulation (DIC) [1-3]. The association between malignancy and thromboembolic phenomena is particularly well-known, first described by Trousseau in 1865, when he characterized migratory superficial thrombophlebitis [4]. Later work by Ziegler in 1888 helped to identify the sterile vegetations arising in chronic inflammatory conditions that would come to be known as NBTE [5]. While poorly understood, the current theory is that a hypercoagulable state induced by inflammation leads to the deposition of sterile vegetations consisting mainly of platelets and fibrin on the heart valves, typically without valvular functional impairment. This may be due to inflammatory cytokines such as tumor necrosis factor and interleukins damaging the valve endothelium, predisposing to thrombus formation in areas with turbulent blood flow, such as across valves [6,7]. In most cases, vegetations are detected on the aortic and/or mitral valves, and commonly observed systemic manifestations typically suggest left-sided disease due to arterial emboli, such as ischemic cerebral infarcts [2]. Right-sided heart involvement is rare, and much more difficult to detect clinically. The majority of such valve involvement is typically diagnosed in the postmortem period [1,8].
Pancreatic adenocarcinoma in particular has demonstrated a strong association with NBTE, although most data are obtained postmortem [9-11]. One autopsy series of 1640 patients showed that NBTE was much more common in patients with this malignancy as compared to other adenocarcinomas (10.34% versus 1.55%; p< 0.05) [9]. Another study of 32 autopsy cases with known marantic vegetations found 20 of them (62.5%) to be malignancy-related, with 3 of 20 (15%) being pancreatic [12]. Many cases of NBTE in the setting of advanced malignancy are asymptomatic and therefore not diagnosed. For example, in one necropsy series of 2041 patients with no clinical evidence of NBTE prior to expiring, 22 had autopsy findings consistent with NBTE, of which pancreatic cancer had the strongest association in cases of concomitant malignancy [13]. As a result, diagnoses in the ante-mortem setting are rare, typically made at the onset of symptoms from thromboembolic disease or valvular compromise [9,14]. These data suggest that asymptomatic NBTE may be more prevalent than reported. This is supported by prospective echocardiographic studies of asymptomatic patients with solid malignancies, which showed the highest association of detected valvular lesions to be with pancreatic cancer (up to 57% of such patients screened) [10,15]. The pathophysiology behind this association is still unclear, but it is thought in part to be due to the excessive secretion of the molecule mucin into the circulation by pancreatic adenocarcinomas [9-11,16]. This molecule’s predilection for selections on endothelial surfaces may be associated with a hypercoagulable microenvironment [17]. This may explain the increased number of hypercoagulable phenomena seen with pancreatic tumors in the body and tail of the pancreas, where mucinsecreting glands are found in higher concentrations [18].
The clinical diagnosis can be challenging, as the disease can be insidious or easily conflated with infectious endocarditis. Many patients are asymptomatic, and are diagnosed only after the onset of symptoms to suggest arterial embolization, such as the acute ischemic cerebral infarctions seen in our patient. The presence of a cardiac murmur is considered nonspecific, especially since NBTE vegetations are traditionally small and do not typically impair valve function [2,19]. The absence of infectious signs and the presence of known malignancy that predisposes to thrombosis should raise concern for NBTE. Bilateral or right-sided heart valve involvement is also more suggestive of NBTE in a patient without risk factors for right-sided endocarditis, such as intravenous drug use [20].
Other findings, such as the pattern of embolization to the brain on diffusion-weighted imaging sequences, may also be helpful. In the setting of NBTE, patients uniformly show a pattern of numerous widely distributed ischemic infarctions of varying sizes in both cerebral hemispheres (as seen in our patient), whereas infectious endocarditis tends to have much more variable findings [21].
The presentation of arterial embolic phenomena is likely due to most NBTE vegetations arising within the left side of the heart, most commonly on the aortic followed by mitral valves [1]. Dual involvement of both the aortic and mitral valves is also seen frequently. These sterile vegetations are known to dislodge and embolize more often than infectious vegetations [22]. Right heart involvement is quite rare; with few reported cases in living patients and most vegetations detected in the postmortem setting [1,2,8,11-13,22-24]. Pulmonary emboli appear to be common findings in NBTE patients, found in as many as 50% of cases, although it is difficult to ascertain if these emboli arise from the right-sided heart valves, or the more distal venous circulation (e.g., from a DVT) [25].
TTE is an ideal first study for the detection of cardiac vegetations, as it is noninvasive and readily available, although a more invasive transesophageal echocardiogram (TEE) is considered superior for detecting valvular vegetations [26,27]. Bubble contrast should be considered if an intra-cardiac defect with right-to-left shunting is suspected as a cause of arterial embolic events; as this was not used during our patient’s study, this remains a possible etiology behind her ischemic strokes. Many patients will not have any overt findings on either study to suggest NBTE, however, so the clinical acumen of the practitioner remains an important part of NBTE diagnosis and management.
The mainstay of treatment is to control the underlying inflammatory cause. In the case of malignancy, surgery, radiation therapy, and chemotherapy may be considered, depending on the extent of disease and the patient’s performance status. This may be difficult to achieve in patients with advanced disease, and a response to therapy may be delayed. Pancreatic cancer in particular is almost always diagnosed in advanced stages, where the treatment goal is mostly palliative [28]. Recent advances in both the diagnosis and treatment of pancreatic cancer, however, have improved survival and result in a higher number of patients developing complications such as NBTE. Our patient was a candidate for chemotherapy prior to the onset of her debilitating neurological deficits. Such a substantial change in outcome raises the question of whether screening for NBTE in high-risk patients would be of benefit despite the condition’s rarity, given that a delayed diagnosis may have severe consequences. Theoretically, early detection and treatment of NBTE in patients with advanced pancreatic cancer may not only reduce morbidity associated with an already grave condition, but also permit administration of life-extending therapies. Further studies are needed to better define this potential benefit, and oncologists treating advanced pancreatic cancer patients should maintain a high index of suspicion to work up and treat patients for NBTE if it is suspected clinically.
This case also raises interesting points of discussion regarding the anticoagulation-based management of malignancy-associated venous thromboembolism (VTE), given our patient’s prior use of rivaroxaban for her DVTs and PEs. Data already suggest that heparin products, particularly low-molecular weight heparins, are superior to coumarins for the prevention of malignancy-associated VTE (hazard ratio: 0.48) [29]. The recent rise of novel oral anticoagulants (NOACs), however, brings with it new opportunities in malignancyassociated VTE therapy. These agents have already demonstrated efficacy in the management of VTE in the general population; rivaroxaban, for example, was shown to be equally effective at treating VTE as compared to enoxaparin and vitamin K antagonists in the EINSTEIN studies [30]. Their relative ease of administration makes them tempting options in cancer patients, for whom quality of life is often a more important consideration. Unfortunately, data assessing NOAC efficacy specifically for malignancy-associated VTE remain limited. Additional work is needed to better define the role of NOACs in this population. In the case of rivaroxaban, studies to assess both primary prophylaxis (NCT02555878) and secondary prophylaxis (NCT01989845) in cancer patients are underway, with eagerly anticipated results.
For NBTE, which fewer studies address due to a paucity of cases, anticoagulation is the most common means of treating the condition; other approaches such as valvular surgery are considered only in select patients [14,20]. Unfractionated heparin is considered to be the most efficacious, but success has been seen with low-molecular weight heparin as well [2,31-33]. Our patient developed NBTE while on active treatment with rivaroxaban for prior DVTs/PEs, and stabilized after her regimen was transitioned to enoxaparin with no further symptom progression. No studies have yet to compare heparin with warfarin and other NOACs such as rivaroxaban for the treatment of this condition, although there is some evidence that warfarin is inferior to heparin at preventing further thromboembolic events in NBTE [2,34]. Thus, heparin products remain essential in the treatment of NBTE. These findings will be important to consider as NOAC use increases and more data regarding their efficacy in malignancyassociated VTE management become available. Whether an earlier transition from rivaroxaban to enoxaparin in our patient could have reduced her morbidity remains an important consideration.
Conclusion
Our patient described above had known metastatic pancreatic adenocarcinoma and presented with new embolic phenomena secondary to NBTE. The unique aspects of this case are possible bilateral cardiac valvular involvement or cardiac septal defects resulting in arterial embolic events in the setting of tricuspid valve endocarditis, occurring while on rivaroxaban therapy. This condition is a significant cause of morbidity and mortality for cancer patients and there are little data available to guide management decisions. It is likely to be more prevalent than currently believed, however, and remains underdiagnosed in high-risk patients. Early recognition and therapy is therefore essential for providing such patients with the best possible outcome.
References
- Eiken PW, Edwards WD, Tazelaar HD, McBane RD, Zehr KJ. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985-2000. Mayo Clinic Proc. 2001; 76: 1204-1212.
- El-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007; 12: 518-523.
- Steiner I. [Nonbacterial thrombotic endocarditis--a study of 171 case reports]. Cesk patol. 1993; 29: 58-60.
- Khorana AA. Malignancy, thrombosis and Trousseau: the case for an eponym. Journal of Thrombosis and Haemostasis. 2003; 1: 2463-2465.
- Ziegler E. Ueber den Bau und die Entstehung der endocarditis chen Efflorescenzen. Ver Kong Inn Med. 1888; 7: 339-343.
- Bick RL. Cancer-associated thrombosis. N Engl J Med. 2003; 349: 109-110.
- Smeglin A, Ansari M, Skali H, Oo TH, Maysky M. Marantic endocarditis and disseminated intravascular coagulation with systemic emboli in presentation of pancreatic cancer. J Clin Oncol. 2008; 26:1383-1385.
- Bhimani AA, Hoit BD. Extensive nonbacterial thrombotic endocarditis isolated to the tricuspid valve in primary antiphospholipid syndrome. J Am Soc Echocardiogr. 2010; 23:107.e5-6.
- Jameson GS, Ramanathan RK, Borad MJ, Downhour M, Korn R, Von Hoff D. Marantic Endocarditis Associated with Pancreatic Cancer: A Case Series. Case Rep Gastroenterol. 2009; 3: 67-71.
- Edoute Y, Haim N, Rinkevich D, Brenner B, Reisner SA. Cardiac valvular vegetations in cancer patients: a prospective echocardiographic study of 200 patients. The American journal of medicine. 1997; 102: 252-258.
- Gonzalez QA, Candela MJ, Vidal C, Roman J, Aramburo P. Non-bacterial thrombotic endocarditis in cancer patients. Acta cardiol. 1990; 46:1-9.
- Waller BF, Knapp WS, Edwards JE. Marantic valvular vegetations. Circulation. 1973; 48: 644-650.
- Llenas-García J, Guerra-Vales JM, Montes-Moreno S, López-Ríos F, Castelbón-Fernández FJ, Chimeno-García J. Nonbacterial thrombotic endocarditis: clinicopathologic study of a necropsy series. Rev Esp Cardiol. 2007; 60: 493-500.
- Tei T, Nomura T, Naito D, Kojima A, Urakabe Y, Enomoto-Uemura S, et al. Effective surgical treatment for controlling the acute heart failure induced by acutely progressed mitral regurgitation with nonbacterial thrombotic endocarditis. Journal of Cardiology Cases. 2010; 20:e59-62.
- Brenner B, Reisner SA, Haim N, Rinkevich D, Edoute Y. Correlation of cardiac valvular vegetations with thromboembolism and plasma d-dimer levels-a prospective echocardiographic study of 200 cancer patients. Fibrinolysis. 1996; 10:16.
- Whang EE, Danial T, Dunn JC, Ashley SW, Reber HA, Lewin KJ, et al. The spectrum of mucin-producing adenocarcinoma of the pancreas. Pancreas. 2000; 21: 147-151.
- Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest. 2003; 112: 853-862.
- Pinzon R, Drewinko B, Trujillo JM, Guinee V, Giacco G. Pancreatic carcinoma and Trousseau's syndrome: experience at a large cancer center. J Clin Oncol. 1986; 4: 509-514.
- Mazokopakis EE, Syros PK, Starakis I K. Nonbacterial thrombotic endocarditis (marantic endocarditis) in cancer patients. Cardiovasc Hematol Disord Drug Targets. 2010; 10: 84-86.
- Borowski A, Ghodsizad A, Cohnen M, Gams E. Recurrent embolism in the course of marantic endocarditis. Ann Thorac Surg. 2005; 79: 2145-2147.
- Singhal AB, Topcuoglu MA, Buonanno FS. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis a diffusion-weighted magnetic resonance imaging study. Stroke. 2002; 33: 1267- 1273.
- Roldan CA, Sibbitt WL, Qualls CR, Jung RE, Greene ER, Gasparovic CM, et al. Libman-Sacks endocarditis and embolic cerebrovascular disease. JACC Cardiovasc Imaging. 2013; 6: 973-983.
- Fink L, Reichek N, Sutton MG. Cardiac abnormalities in acquired immune deficiency syndrome. The Am J Cardiol. 1984; 54: 1161-1163.
- Lopez JA, Fishbein MC, Siegel RJ. Echocardiographic features of nonbacterial thrombotic endocarditis. Am J Cardiol. 1987; 59: 478-480.
- Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Noninfective valvular masses: review of the literature with emphasis on imaging techniques and management. Am Heart J. 1996; 131: 1175-1183.
- Roldan CA, Qualls CR, Sopko KS, Sibbitt WL. Transthoracic versus transesophageal echocardiography for detection of Libman-Sacks endocarditis: a randomized controlled study. J Rheumatol. 2008; 35: 224-229.
- de Bruijn SF, Agema WR, Lammers GJ, van der Wall EE, Wolterbeek R, Holman ER, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006; 37: 2531-2534.
- Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999; 189: 1-7.
- Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Eng J Med. 2003; 349: 146-153.
- Prins MH, Lensing AW, Brighton TA, Lyons RM, Rehm J, Trajanovic M, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomized controlled trials. The Lancet Haematology. 2014; 1: e37-46.
- Shatila W, Rizkallah A, Aldin ES, Tfayli A. Nonbacterial thrombotic endocarditis as the sole manifestation of stage IV gastric cancer: a case report. J Med Case Rep. 2014; 8: 267.
- Rogers LR, Cho ES, Kempin S, Posner JB. Cerebral infarction from non-bacterial thrombotic endocarditis: clinical and pathological study including the effects of anticoagulation. Am J Med. 1987; 83: 746-756.
- Lopez JA, Ross RS, Fishbein MC, Siegel RJ. Nonbacterial thrombotic endocarditis: a review. Am Heart Journal. 1987; 113: 773-784.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141: e576S-600S.