
Research Article
Ann Hematol Oncol. 2016; 3(3): 1081.
Thalidomide-Dexamethasone Induction Followed by Autologous Transplantation in Young Patients with Multiple Myeloma
Kmira Z¹*, Ikbel BH¹, Houneida Z², Monia Z¹, Hela Z³, Yosra BY³ and Abderrahim K¹
¹Department of Clinical Hematology, Farhat Hached University Hospital, Tunisia
²Department of Radiology, Farhat Hached University Hospital, Tunisia
³Department of Rheumatology, Farhat Hached University Hospital, Tunisia
*Corresponding author: Zahra Kmira, Department of Clinical Hematology, Farhat Hached University Hospital, Sousse, Avenue Ibn Eljazzar Sousse 4000, Tunisia
Received: April 04, 2016; Accepted: May 31, 2016; Published: June 02, 2016
Abstract
Aim: Thalidomide plus dexamethasone (TD) has shown encouraging results in newly diagnosed multiple myeloma (MM) in small, uncontrolled trials. The aim of the present retrospective study was to compare the efficacy and toxicity of TD and melphalan and prednisone (MP) in young MM patients.
Methods: In this retrospective study, patients with symptomatic MM were treated with TD (arm A) or MP (arm B). Patients in arm A received oral thalidomide 200 mg daily associated to oral dexamethasone 20 mg/m² on days 1-4, 9-12, and 17-20 of the first and the third cycle and on days 1-4 of the second cycle (28-day cycles). Patients in arm B received six 30-day cycles of melphalan at the dose of 0.25 mg/kg/day on days 1-7 of each cycle associated to prednisone at the dose of 2 mg /kg/day on days 1-7 of each cycle. Thirteen patients in the arm A received high dose therapy (HDT) with autologous stem cell transplantation (ASCT).
Results: Thirty six patients were enrolled: 24 patients in the arm A and 12 patients in the arm B. The response rate with TD was significantly higher than with MP (77.3% versus 50%, respectively). The overall survival (OS) and the event free survival (EFS) at 18 months were higher in TD versus MP (76% v 37%; p= 0.028 and 63% v 33%; p= 0.019, respectively). The OS and the EFS at 18 months were higher in the arm autograft versus the arm no-autograft (90% versus 44.7%; p= 0.004 and 61.7% versus 40%; p =0.01, respectively). No statistically significant difference was observed between arms TD and MP in terms of neutropenia, anemia, thrombocytopenia, deep vein thrombosis and peripheral neuropathy (P >0.05).
Conclusion: TD demonstrates significantly superior response rates in newly diagnosed myeloma compared with MP. ASCT improves outcome in young MM patients.
Keywords: Multiple myeloma; Melphalan; Prednisone; Thalidomide; Dexamethasone
Introduction
Multiple myeloma (MM) is a malignant plasma-cell proliferative disorder that accounts for approximately 10% of hematologic malignancies [1]. For many years, melphalan plus prednisone (MP) had remained the standard therapy for this disease. Response rates with this therapy are approximately 50%; median survival is approximately 3 years [2-3]. First-line treatment in MM aims primarily at high response rates and early reduction of tumor burden, achieved with least possible toxicity to bone marrow stem cells, since high dose therapy (HDT) with autologous stem-cell transplantation (ASCT) in eligible patients is by now the only therapeutic strategy that prolongs OS [4-6]. So, vincristine, doxorubicin, and dexamethasone (VAD) and VAD-like regimens including vincristine, liposomal doxorubicin and dexamethasone (VAD-doxil), have replaced MP and been widely accepted as first-line treatment in MM during the last two decades, including early, objective responses in 55-67% of patients [7]. Thalidomide was firstly explored for the treatment of advanced and refractory MM by Singhal et al. in 1999 with a response rate of 25% to 35% [8]. The rationale for using this drug in patients with progressive MM relied upon the notion that increased bone marrow angiogenesis correlates with advanced phases of MM [9] and on data from previous studies showing the antiangiogenic activity of thalidomide in vitro [10]. In combination with dexamethasone, response rates increase to approximately 50% in relapsed refractory disease [11]. Three phase II trials have been conducted with the thalidomide plus dexamethasone (TD) combination in newly diagnosed MM. In the Mayo Clinic trial, 50 patients were treated and 64% responded to therapy [12]. Similar response rates were seen in the M.D. Anderson clinical trial and the Italian clinical trial, respectively [13,14]. As a result of these phase II trials, the use of TD has increased significantly in standard practice. Some studies have reported the superiority of TD compared with VAD based on short-term response rates [15,16].
The goal of this study was to compare the response rate, the overall survival (OS), and event free survival (EFS) of TD followed by ASCT versus MP in young patients (< 65 years) with newly diagnosed MM.
Materials and Methods
Patients
It is a retrospective study including 36 patients with newly diagnosed MM treated in Clinical Hematology department from March 2007 to December 2011. Patients 18 to 65 years of age with secretory and non-secretory MM Durie-Salmon stage II to III were eligible (Table 1). Exclusion criteria were asymptomatic stage I, systemic amyloid light chain amyloidosis, neuropathy grade ≥ 2, active malignancy during the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma, HIV positivity, serum bilirubin ≥ 30 μmol/L or aminotransferases ≥ 2.5 x normal level, pregnancy and severe psychosis. Patients with renal impairment were not excluded.
Characteristics
Total number of patients
Treated with TD
Treated with MP
Numbers
36
24
12
Sex, M/F
13/23
10/14
3/9
Age
Median (range) years
55,5(38-65)
52 (38-61)
61,5(60-65)
Monoclonal component
IgG/ IgA/ k/ λ
21/8/5/2
13/7/4/0
8/1/1/ 2
Durie-Salmon stage
I/II/III
A/B
0/10/26
29/7
0/5/19
19/5
0/ 5/ 7
10/2
ISS stage
1/2/3
7/17/12
4/13/7
3/ 4/ 5
Autologous stem-cell transplantation
13
13
-
Abbreviations: TD: Dexamethasone-Thalidomide; MP: Melphalan- Prednisone; ISS: International Staging Score.
Table 1: Patients’ characteristics.
Treatments
Induction therapy was TD in 24 patients and MP in 12 patients. In the treatment arm of TD, patients received 200 mg/day of thalidomide for three 28-day cycles associated to dexamethasone at a dose of 20mg/m² orally on days 1-4, 9-12, and 17-20 of the first and the third cycle and on days 1-4 of the second cycle. In the arm of MP, patients received six 30-day cycles of melphalan at the dose of 0.25 mg/kg/day on days 1-7 of each cycle associated to prednisone at the dose of 2 mg /kg/day on days 1-7 of each cycle. Thromboprophylaxis by aspegic 100 mg/day was used in all patients in the TD arm. No antibioprophylaxis was used in the two arms.
After induction therapy by TD, patients received a single infusion of cyclophosphamide 4 g/m² followed by daily administration of subcutaneous G-CSF at the dose of 5 μg/kg from the fifth day after the injection of cyclophosphamide until the peripheral stem cell harvest. Only 13/24 patients (54%) received high-dose melphalan (200 mg/m²) followed by stem-cell transplantation because of refusal of the autograft, death during induction, and unspecified reasons respectively in 4, 2 and 5 cases. No maintenance treatment was given in the 2 treatment arms and all patients have received bisphosphonates for two years.
Response criteria
Response, relapse and progression were assessed according to the international Myeloma Working Group (IMWG) criteria [17]. Complete response (CR) was defined as negative serum and urine immune fixation, < 5% plasma cells in a bone marrow aspirate as well as disappearance of soft tissue plasmacytomas and no increase in lytic bone lesions. Very Good partial response (VGPR) was defined as detectable serum and urine M-protein by immunofixation but not on electrophoresis or 90% or greater reduction in serum M-protein plus urine M-protein level < 100 mg per 24 h. Partial response (PR) was defined as a decrease of serum M-protein in ≥ 50%, 24-hour urinary light-chain excretion by ≥ 90% or to < 200 mg, and reduction of extra medullary plasmacytomas in ≥ 50%. Stable disease was defined as the absence of criteria for CR, VGPR, PR or progressive disease. Responses should be maintained for a minimum of 6 weeks. Relapse from CR was defined as reappearance of serum or urinary paraprotein on immunofixation, development of new extramedullary plasmacytomas, increase in size, or developing of new lytic lesions or hypercalcemia. Progressive disease required an increase in serum M-protein by >25% with an absolute increase of at least 5 g/L or increase in urine M-protein by >25% and also an absolute increase ≥200 mg/24 h, and/or the appearance of soft-tissue plasmacytomas, new lytic bone lesions, or hypercalcemia.
Statistical analysis
Data were analyzed with the Statistical Package for the Social Sciences (SPSS). The Kaplan–Meier method was used to estimate time-to-event distributions, and stratified log-rank tests and Cox models, at a two-sided alpha level of 0.05, were used for betweengroup comparisons of time-to-event end points.
Results
Patients’ characteristics
The distribution of the patients’ baseline characteristics was comparable among the two treatment groups (Table 1).
Response and survival
Overall response rate (ORR) was significantly better in TD regimen versus MP regimen (77.3% versus 50%). CR was achieved in 3 patients (13.7%) in TD arm and not achieved in MP arm (Table 2). A total of 13 (54%) patients in arm TD, 2 in CR and 11 in non- CR, preceded to ASCT after completion of the fourth cycle. Posttransplant CR was achieved in 4 (36.5%) of the patients that entered ASCT in the non-CR state (Table 3) (Figure 1).
Response
Number patients (%)
TD (n= 24)
MP(n=12)
Overall response
17 (77,3)
4 (50)
Complete response
3 (13,7)
-
Very Good partial response
14(63,6)
2(25)
Partial response
-
2 (25)
Stable disease
5 (22,7)
4(50)
Not available (early death)
2 (8,3)
4 (33,3)
Table 2: Response rates of TD versus MP.
Response
Before autograft
Number patients (%)
After autograft
Number patients (%)
Complete response
2 (15,4)
6(46,2)
Very Good partial response
-
3(23)
Partial response
8 (61,5)
4(30,8)
Stable disease
3 (23,1)
-
Table 3: Clinical response before and after autologous of stem-cell transplantation.
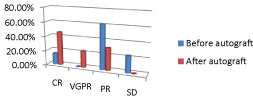
Figure 1: The improvement of overall response rate after autograft.
The overall survival (OS) at 18 months was 76% in TD arm and 37% in arm MP arm (p= 0.028) (Figure 2). The event free survival (EFS) at 18 months was 63% in TD arm and 33% in MP arm (p= 0.019) (Figure 3). The OS and the EFS at 18 months were higher in the arm autograft versus the arm no-autograft (90% versus 44.7%; p= 0.004 and 61.7% versus 40%; p= 0.01, respectively) (Figures 4,5).
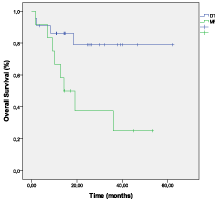
Figure 2: OS after induction therapy with TD versus MP.
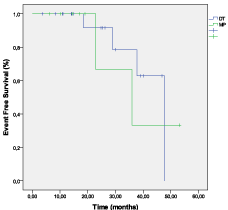
Figure 3: EFS after induction therapy with TD versus MP.
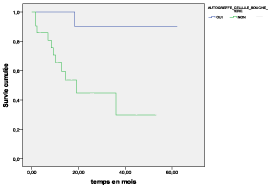
Figure 4: The impact of autologous stem cell transplantation on OS.
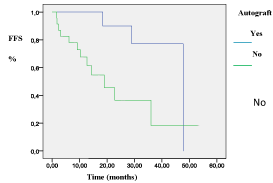
Figure 5: The impact of ASCT on EFS.
Adverse Events
Overall toxicities are displayed in (Table 4). No statistically significant difference was observed between arms TD and MP in terms of neutropenia, anemia, thrombocytopenia, deep vein thrombosis and peripheral neuropathy (P >0.05).
TD (24 patients)
n (%)
MP (12patients),
n (%)
P value
Neutropenia
Grade 1-2
Grade 3-4
1(4%)
0
0
1(12%)
0.58
Anemia
Grade 1-2
Grade 3-4
0
2(8%)
0
0
0.58
Thrombocytopenia
Grade 1-2
Grade 3-4
0
0
1(12%)
0
0.16
Deep vein thrombosis
2(8%)
0
0.58
Peripheral neuropathy
0
0
Table 4: Toxicity of TD versus MP
Discussion
The present study confirms the superiority of TD over MP in terms of objective response rate (77.3% versus 50%) in newly diagnosed myeloma patients. More importantly, the present study demonstrates that the depth of response is significantly higher with TD; 77.3% of patients achieved CR or VGPR with TD compared with only 25% with MP. Achievement of CR and VGPR are the best predictors of long-term outcome in myeloma [18]. As clearly shown, the significantly improvement in response rates achieved with TD does translate into longer OS and EFS. In some studies, high-dose therapy with ASCT has resulted in significantly prolonged survival compared with conventional-dose chemotherapy [5-6 ]. In our study, we found that post-transplant CR was achieved in 36.5% of the patients that entered ASCT in the non-CR state and OS and EFS were higher in the arm of TD. Although most of our patients did not need to interrupt therapy or to reduce the doses, thalidomide was not without toxicity. In addition to the most common side effects, which were generally mild and well manageable, deep vein thrombosis emerged as the most troublesome adverse event associated with primary thalidomide dexamethasone therapy. The frequency of deep vein thrombosis among patients with advanced and refractory MM was reported to be less than 5% with the use of thalidomide alone and increased substantially, up to 16%, when thalidomide was combined with chemotherapy regimens containing doxorubicin [11- 19]. A high risk of deep vein thrombosis, ranging from 10% to 28%, was also observed among patients with de novo MM who received thalidomide combined with dexamethasone or with chemotherapy regimens that included doxorubicin and dexamethasone [20-22]. The rate of deep vein thrombosis observed in our study (8%) was in the range reported in previous studies.
Although TD has emerged as an oral alternative to intravenous induction regimens for myeloma, more effective and safer regimens are needed. Recent studies show that lenalidomide, an analog of thalidomide, may be safer and more effective than thalidomide. A combination trial with lenalidomide plus dexamethasone has already shown improved activity with lower toxicity in a phase II clinical trial [23]. Large phase III trials are ongoing in the United States headed by Eastern Cooperative Oncology Group (ECOG) and the Southwest Oncology Group to investigate the role of lenalidomide plus dexamethasone in newly diagnosed multiple myeloma. Similarly, high activity has been observed with bortezomib based induction in several phase II trials.
We conclude that the combination of thalidomide plus dexamethasone is a feasible and active regimen in the treatment of MM. Furthermore, additional important issues that need to be addressed in future clinical trials include the role of thalidomide in combination with chemotherapy or with proteasome inhibitors for induction of remission before autologous stem cell transplantation and/or as consolidation of remission or maintenance therapy after autologous transplantation.
References
- Sirohi B, Powles R. Multiple myeloma. Lancet. 2004; 363: 875-887.
- Alexanian R, Haut A, Khan AU, Lane M, McKelvey EM, Migliore PJ, et al. Treatment for multiple myeloma, combination chemotherapy with different melphalan dose regimens. JAMA. 1969; 206: 1680-1685.
- Myeloma Trialists’ Collaborative Group: Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: An over view of 6,633 patients from 27 randomized trials. J Clin Oncol. 1998; 16: 3832-3842.
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004; 351: 1860-1873.
- Attal M, Harousseau JL, StoppaAM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996; 335: 91-97.
- Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003; 348: 1875-1883.
- Alexanian R, Barlogie B, Tucker S. VAD- based regimens as primary treatment for multiple myeloma. Am J Hematol. 1990; 33: 86-89.
- Singhal S, Mehta J, DesikanR, Ayers D, Roberson P, Eddlemon P. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999; 341: 1565-1571.
- Vacca A, Ribatti D, Roncali L, Ranieri G, Serio G, Silvestris F, et al. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol. 1994; 87: 503-508.
- D’Amato RJ, Lougran MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994; 91: 4082-4985.
- Dimopoulos MA, Anagnostopoulos A, Weber D. Treatment of plasma cell dyscrasias with thalidomide and its derivatives. J Clin Oncol. 2003; 21: 4444-4454.
- Rajkumar SV, Hayman S, Gertz MA, Dispenzieri A, Lacy MQ, Greipp PR, Geyer S, et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol. 2002; 20: 4319-4323.
- Weber D, Rankin K, Gavina M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J C Oncol. 2003; 21: 16-19.
- Cavo M, Zamagni E, Tosi P, Cellini C, Cangini D, Tacchetti P, et al. First-line therapy with thalidomide and dexamethasone in preparation for autologous stem cell transplantation for multiple myeloma. Haematologia . 2004; 89: 826-831.
- Cavo M, Zamagni E, Tosi P, Cellini C, Cangini D, de Vivo A, et al. Superiority of thalidomide and dexamethasone over vincristine-doxorubicin-dexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005; 106: 35-39.
- Jimenez-Zepeda V, Dominguez-Martinez V. Vincristine, doxorubicin, and dexamethasone or thalidomide plus dexamethasone for newly diagnosed patients with multiple myeloma? Eur J Haematol. 2006; 77: 239-244.
- Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006; 20: 1467-1473.
- Attal M, Harousseau J-L, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003; 349: 2495-2502.
- Cavenagh JD, Oakervee H. Thalidomide in multiple myeloma: current status and future prospects. Br J Haematol. 2003; 120: 18-26.
- Cavo M, Zamagni E, Cellini C, Tosi P, Cangini D, Cini M, et al. Deep-vein thrombosis in patients with multiple myeloma receiving first-line thalidomide- dexamethasone therapy. Blood. 2002; 100: 2272-2273.
- Osman K, Comenzo R, Rajkumar SV. Deep venous thrombosis and thalidomide therapy for multiple myeloma. N Engl J Med. 2001; 344: 1951-1952.
- Zangari M, Anaissie E, Barlogie B. Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood. 2001; 98:1614-1615.
- Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005; 106: 4050-4053.