
Research Article
Ann Hematol Oncol. 2016; 3(4): 1087.
Effects of Gasoline Poisoning on Some Hematological Parameters
Seriki SA*, Adebayo OF, Shafe MO and Bivan ZK
Department of Human Physiology, Bingham University, Nigeria
*Corresponding author: Seriki Adinoyi, Department of Human Physiology, College of Medicine Bingham University, Nigeria
Received: May 31, 2016; Accepted: June 27, 2016; Published: June 29, 2016
Abstract
Background: Gasoline, also known as Premium Motor Spirit (PMS) is a major product of crude oil used in Nigeria and other parts of the world for fueling engines. Workers with industries and stations where this product is refined or sold to motorists are exposed to its poisoning either by inhalation or diet contamination. The present study investigated the effects of inhaled gasoline and gasoline-contaminated diets on some hematological parameters of Wister rats after three weeks (21 days) of chronic exposure.
Method: Thirty (30) rats used for the study were grouped into three (n=10). Group 1 served as the control group and was given normal rat feeds and water, Group 2 was exposed to gasoline inhalation, and Group 3 was treated with gasoline-contaminated feeds. Blood was collected through retro-orbital puncture on the 22nd day (i.e., 24 hours after the final day of exposure).
Result: Gasoline caused significant increase (P< 0.05) in total white blood cell (WBC) count. Though not significant, there was a decrease in the red blood cell (RBC) and packed cell volume (PCV) in the treated groups when compared with the control group. The protracted exposure to gasoline also caused significant increase in platelet count (PLT).
Conclusion: Protracted exposure to gasoline (either by inhalation or contamination) is generally detrimental to blood parameters and the general health of an individual. Workers engaged with gasoline companies should therefore find a way of reducing chronic exposure to gasoline.
Keywords: Gasoline; Red blood cell; White blood cell; Platelet; Packed cell volume
Introduction
Hematology is the science of the blood, its formation and functions. It encompasses the study of blood cells and coagulation, which includes analyses of the concentration, structure, and function of cells in blood; their precursors in the bone marrow; chemical constituents of plasma or serum intimately linked with blood cell structure and function; and function of platelets and proteins involved in blood coagulation.
Blood is a specialized body fluid that delivers necessary substances to the body’s cells such as nutrients and oxygen. It also transports waste products away from those same cells. Blood is the most important body fluid that governs vital functions of the body like respiration, circulation, excretion, osmotic balance and transport of metabolic substance. Circulation of the blood within the cardiovascular system is essential for transportation of gases, nutrients, minerals, metabolic products and hormones between different organs [1].
Blood is composed of two components, namely; Plasma (55%) and Blood cells (45%).Plasma is a straw color clear liquid part of the blood. It contains 91-92% of water and 8-9% solids, while the blood cells are of three main types namely; Red Blood Cells (RBC) or Erythrocytes, White Blood Cells (WBC) or Leukocytes, and Platelets or Thrombocytes [2].
Red Blood Cells are the non-nucleated formed elements in the blood, and the count normally, ranges between 4.2-5.9 million/mm³ in humans. It has a shape of biconcave disc, about 7μm in diameter and 2.2μm thick. Their unique shape relates to their function of transporting oxygen. The red color of the cells is due to the presence of hemoglobin. They are mainly formed in the hematopoietic stem cells of the bone marrow by erythropoiesis and have a life span of about 120 days.
White Blood Cells, on the other hand, are the colorless and nucleated formed elements of the blood. They are larger in size and lesser in number when compared with the red blood cells. WBCs are either granulocytes or agranulocytes. Generally they are; Neutrophils (54-62%), Eosinophils (1-3%), Basophils (less than 1%), Monocytes (3-9%), and Lymphocytes (25-33%). The Life span of each white blood cell and functions differ. The white blood cell count ranges between 5000-10000/mm³ in human.
Platelets are the formed elements of the blood. They are small colorless, non-nucleated and moderately refractive bodies. Platelet count ranges between 100000-140000/mm³ [3].
Other blood parameters include packed cell volume (PCV) which is an important parameter used to express the proportion of red blood cell in percentage and this is significant in the diagnosis and treatment of conditions such as anemia. The normal range is 38-42% in females and 40-45% in males. Also, there are some other blood indices measured for the purpose of diagnosis. They include;
1. Mean corpuscular volume
2. Mean corpuscular hemoglobin
3. Mean corpuscular hemoglobin concentration
4. Color index.
Blood parameters are probably the more rapid and detectable variations under stress and fuel assessing the health condition [4]. The importance of hematological parameters in clinical biochemistry, population genetics and medical anthropology is well established. Speculations have shown that they may be used as valuable indicators of disease or stress in animals [5].
The use of gasoline in the industries and homes has rapidly increased in the recent times. In the course of usage, individuals are frequently exposed to pollutants from it in both outdoor and indoor environments. However, the major route of exposure is inhalation by workers during production and distribution of the fuel, and by the general public during refueling at service stations [6].
Gasoline, one of the fractionated products of crude oil, is widely used as fuels for automobiles and some electricity generating machines. It is known to be a very volatile liquid, with several organic and inorganic constituents. Gasoline vapour may be derived from direct evaporation of liquid gasoline [7].
Exposures to these pollutants are common in the refineries, oil fields, refueling stations, petrochemical industries, motor mechanical workshops, and traffic-congested areas. However, the population at greater risk of frequent exposure includes those occupationally exposed [8]. It has been reported that the oil drillers, refinery workers, petrochemical workers, refuel station attendants and motor mechanics suffer the greater risk of chronic exposures to petroleum pollutants [9], and are therefore more likely to come down with blood related abnormalities.
Materials and Methods
Materials
Gasoline was purchased from Nigerian National Petroleum Commission (NNPC) filling station located in Karu. And was stored in a non-corrosive container and used during the course of the experiment. Other materials include: Steel cages, Test tubes, EDTA bottles- heparinised bottle, Cotton wool, Capillary tube, Dissecting kit, Hand gloves, Masking tape
Experimental animals
Wistar Albino rats weighing between 100g and 130g were used in this experiment. The rats obtained from the Animal house of National Veterinary Research Institute, Vom, Jos town Plateau State, Nigeria, were kept at the Animal House of Bingham University, Karu, Nassarawa State to acclimatize for three weeks before the experiment.
They were housed in well ventilated stainless-steel cages at room temperature (24±2°C) in hygienic condition under natural light and dark schedule and were fed on standard laboratory diet. Food and water were given ad libitum.
Experimental design
The rats were divided into three (3) groups of ten rats each. The LD50 value of petrol was obtained as 127 mL/kg body weight of rats (Figure 1).
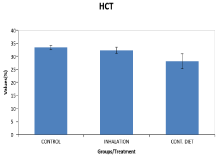
Figure 1: Bar Chart Showing Hematocrit (Mean±Sem).
Group I (Control): Rats in this group were neither exposed to gasoline vapour nor treated with gasoline-contaminated feed.
Group II (Inhalation): Rats in this group were exposed to gasoline vapor (8hours per day) for the 21 days period of the experiment. (Workers in industries in Nigeria run shift-duties of about 8 hours per shift in a day).
Group III (Feed): Rats in this group were treated with gasoline contaminated feed daily. (Workers working with gasoline companies have their food contaminated with the product while on duty).
Determination of LD50
The LD50 was determined using Lorke’s method. This method has two phases, phase 1 and phase 2.
Phase 1: In this phase, 9 animals were used. The animals were divided into 3 groups of 3 animals each. Each group of animals were administered different doses (10, 50 and 100 ml/kg) of the gasoline. The animals were placed under observation for 24 hours to monitor their behavior.
Phase 2: In this phase, 3 animals were used; they were distributed into 3 groups of one animal each. They were administered higher doses (140, 160 and 180 mi/kg) of the gasoline. The animals were then observed for 24 hours for behavior as well as mortality.
LD50 = v (D0 × D100)
Where D0 = Lowest dose that gave no mortality (100 ml/kg)
D100 = Highest dose that produced mortality (160 ml/kg)
LD50 = v (100 ×160)
= v (16000)
= 126.5 ≈ 127 ml/kg.
Exposure to gasoline vapor
The animals in Group II were exposed to gasoline vapour in an exposure chamber. The rats were placed inside the chamber with a 1000ml bottle filled with 500ml gasoline, and perforated at different parts of the top for easy diffusion of ungraded concentrations of the vapor generated from direct evaporation of liquid gasoline. The test animals were allowed to inhale the evaporating vapour in the chamber during the exposure period. An exposure period of 8 hours daily was adopted for 3 weeks.
Treated with gasoline contaminated feed
The animals in Group III were treated with gasoline contaminated feed. Calculated volume of the gasoline was mixed with measured amounts of animal feed and the mixture was compacted with water to form a compact mass. The feed was then moulded into small balls, air-dried and stored in a well-labelled sack to last for the duration of the test period.
Hematological study
At the end of the period of administration, blood for hematological study was collected using the orbital technique [10] into sample bottles containing ethylene diamine tetra acetic acid (EDTA). Immediately after blood collection, the sample bottle was gently shaken to mix up the blood with the EDTA to prevent clotting or lysing of the blood.
Determination of hematological parameters
The Automated Hematologic Analyzer (Sysmex KX – 21) was used to analyze the hematological parameters; PCV, WBC, RBC, and PLT. The analyses were carried out based on standard methods [11].
Statistical analysis
The data was tested by one-way analysis of variance (ANOVA) using the statistical package for social sciences (SPSS) software application (version 20). The multiple comparisons were made using the Post hoc test. The accepted level of significance was set at p < 0.05. Results were reported as means and standard error of means.
Results and Discussion
Results show that there was significant increase in the White Blood Cell (WBC) count in the groups treated with gasoline vapor and contaminated feeds when compared with the control group (p< 0.05). There was a decrease in Red Blood Cell (RBC) counts in the treated groups, which however was not significant when compared with the control group. Packed Cell Volume (PCV) decreased significantly with the contaminated group, but the decrease was insignificant in the inhalation group, when both were compared with the control. The gasoline also caused significant increase in platelet count (PLT) (Thrombocytosis) (Table 1).
CONTROL
INHALATION
CONTAMINATED DIET
RBC (×106/μL)
6.18±0.16
5.89±0.27
5.32±0.57
WBC (×103/μL)
7.47±0.48
9.77±0.41*
9.61±0.73*
PCV (%)
33.43±0.77
32.33±1.14
28.14±2.88*
PLT (×103/μL)
344.70±18.7
456.3±29.70*
366.20±36.03
Key: the asterisk (*) means p< 0.05.The values are represented using Mean ± SEM s indicated in the table.
Table 1: Hematological values.
Gasoline caused significant increase in total white blood cell (WBC) count which could be as a result of the body’s defense mechanism trying to protect the body from being vulnerable to infections having been poisoned by a foreign body (gasoline). Liver cells released into blood due to hepatocelluar damage from gasoline poisoning may have sent signals to the immune effectors cells to provide the body with immunity against infections. This generally is the function of the white blood cells which are the 1st line of defense when the body is vulnerable to infections and other conditions detrimental to the proper functioning of the body system.
There was a decrease in the red blood cell (RBC) and packed cell volume (PCV) in both treated groups (those fed with contaminated feeds and those that inhaled) when compared with the control. This agrees with previous findings that anemia results from poisoning due to reduction in the level of oxygen that would be carried to the tissue as well as the level of carbon dioxide returned to the lungs [12]. Gasoline also caused reduction in the rate of erythropoiesis, implying that gasoline has the potential to inhibit erythropoietin release from the kidneys to enhance erythropoiesis.
It also caused significant increase in platelet count (PLT) (Thrombocythemia and Thrombocytosis) - reactive thrombocytosis.
Thrombocytosis is a condition in which one’s blood has a higher than normal number of platelets. Platelets (thrombocytes) are blood cell fragments; they’re made in your bone marrow along with other kinds of blood cells. A normal platelet count ranges from 150,000 to 450,000 platelets per micro liter of blood. The gasoline may have catalyzed the activity of the bone marrow to produce more platelets
Conclusion
Gasoline, taken in by ingestion (contaminated feeds) or by inhalation is detrimental to blood parameters; it could cause anemia, make the body vulnerable to infections, cause thrombocytosis and Thrombocythemia (restricted blood flow due to platelet formation), a condition that can cause severe ill health and death. Thus, protracted exposure to gasoline is unhealthy to the general wellbeing of an individual and should therefore be discouraged. Workers that deal directly with gasoline must find means of reducing intake (either by inhalation or contamination).
References
- Baynes WJ, Dominiczak HM. Medical Biochemistry. Second Edition. Philadelphia: Elsevier Mosby Ltd: 2005.
- Sembulingam P, Sembulingam A. Essentials of Medical Physiology: Blood. Sixth edition. India: Jaypee brothers: 2009: 56-98.
- Fox S. Human Physiology. Eighth edition. Boston: The McGraw-hill and companies: 2004.
- Hymavathi V, Rao LM. Effect of sub lethal concentrations of lead on the hematology and biochemical constituents of Channa punctatus. Bulletin of Pure and Applied Sciences. 2000: 19A: 1-5
- Calabrese, AL, Thurberg FP, Dawson MA, Wenzloff DR. Sub lethal physiological stress induced by cadmium and mercury in winter flounder Pseudopleuronectesamericanus. In sub lethal effect of toxic chemicals (eds.). JH. Koeman J.J.T.W.A. Strik. Elsevier, Scientific Co. Amsterdam, 1975; 15-21.
- Wixtrom RN, Brown SL . Individual and population exposures to gasoline. J Expo Anal Environ Epidemiol. 1992; 2: 23-78.
- Carballo M, Nigro ML, Fraga I, Gadano A. Ethylene oxide: cytogenetic and biochemical studies in persons occupationally exposed. Environ Mol Mutagen. 1994; 23: 7-12.
- Raabe GK, Wong O . Leukemia mortality by cell type in petroleum workers with potential exposure to benzene. Environ Health Perspect. 1996; 104: 1381-1392.
- EHC 20; selected petroleum products. In; Environmental Health Criteria 20, United Nations Environment Programme, the International Organization and World Health Organization. Geneva. 1982; 5-8.
- Stone SH. Method for obtaining venous blood from the orbital sinus of the rat or mouse. Science. 1954; 119: 100.
- Dacie JV, Lewis SM, Practical Haematology, 5th edition. New York: Churchill Livingstone: 1975.
- Sas B. Secondary copper deficiency in cattle caused by molybdenum contamination of fodder: a case history. Vet Hum Toxicol. 1989; 31: 29-33.