
Review Article
Ann Hematol Oncol. 2016; 3(5): 1090.
The Management of Spinal Cord Compression in Multiple Myeloma
Sen E¹ and Yavas G²*
¹Department of Medical Oncology, Selcuk University, Turkey
²Department of Radiation Oncology, Selcuk University, Turkey
*Corresponding author: Güler Yavas, Department of Radiation Oncology, Selcuk University, Faculty of Medicine, Konya, Turkey
Received: May 13, 2016; Accepted: July 09, 2016; Published: July 12, 2016
Abstract
Multiple myeloma (MM) is a hematopoietic disorder which is characterized by accumulation of monoclonal plasma cells with the most common localization being the spine. Spinal cord compression (SCC) occurs approximately 5% of patients with the diagnosis of MM. Signs and symptoms of SCC include pain, motor defects, sensory deficits; and bowel and bladder dysfunction. Magnetic resonance imaging or computed tomographic myelography of the entire spine must be performed immediately if this complication is suspected. Prompt diagnosis and immediate treatment are critically important in the preservation of neurological function in patients with SCC. The goals of treatment for patients with SCC are pain control, avoidance of complications from local disease progression, and the preservation or improvement of neurologic functioning. The choice of definitive treatment must be appropriate to the patient’s burden of disease, life expectancy, and values. For patients with SCC and either neurologic symptoms or substantial the cal sac compression by imaging, corticosteroids should be an integral component of the initial management. The choice of modality for definitive treatment depends on many factors, including the presence of absence of spinal instability, the degree of spinal cord compression, and the relative radio sensitivity of the tumor. Surgery and radiotherapy are the primary approaches to treat tumor compressing the spinal cord. Systemic therapy such as chemotherapy agents with steroids and either proteasome inhibitors or immunomodulatory drugs, with or without high-dose chemotherapy and stem cell transplantation work rapidly and can be used instead of radiation in selected patients if there is minimal neurologic deficit.
Keywords: Multiple myeloma; Radiation therapy; Spinal cord compression; Surgery
Abbreviations
BKP: Balloon kyphoplasty; CT: Computed Tomography; EBRT: External Beam Radiotherapy; MM: Multiple Myeloma; MBD: Myeloma Bone Disease; MRI: Magnetic Resonance Imaging; PV: Percutaneous Vertebroplasty; RT: Radiation Therapy; SCC: Spinal Cord Compression; SMD: Spinal Myeloma Disease; SINS: Spine Instability Neoplastic Score; SBRT: Stereotactic Body Radiotherapy
Introduction
Multiple myeloma (MM) is a hematological malignancy characterized by accumulation of monoclonal plasma cells. It accounts for approximately 1 % of all cancers and represents about 10 % of all hematological malignancies [1]. The annual incidence is 4.3 per 100,000 [2]. The American Cancer Society estimates that 26,850 new cases of MM will be diagnosed in the US in 2015 [3]. MM is slightly more common in males than females [4]. The median age at diagnosis is 66 years. Only 2% of patients are younger than 40 years of age [5]. MM is an almost incurable disease. Current advances have led to increased survival [6], although this is still much shorter than for other mature B-cell malignancies, such as follicular lymphoma and chronic lymphocytic leukemia [7].This discrepancy may be due to the development of myeloma bone disease (MBD), which affects approximately 60% of patients rising to 80-90% at some stage of their disease [8]. Typically, the disease involves the bone marrow and breaks through the cortex, invading the surrounding tissue. MBDassociated morbidities include pain, pathological fractures, spinal cord compression and hypercalcemia [7-9].
The spine is the most affected skeletal organ, and single or multiple spinal lesions are due to bone destruction caused by MM. Spinal myeloma disease (SMD) is present in up to 60% of patients at diagnosis which may or may not be symptomatic [5]. SMD is most commonly indicated by the presence of lytic lesions or generalized osteopenia. Most lesions occur in the vertebral bodies but they can also be found in other parts of the vertebral columna including the facets, pedicles and transverse/spinous processes [10]. Affected vertebral bodies may become weakened with progressive bone destruction and eventually collapse, resulting in a vertebral compression fracture. Vertebral compression fractures are causing spinal injury. Spinal cord compression can also result from direct extension of tumor into the epidural space.
The spinal cord compression (SCC), which is an oncologic emergency that can cause pain and potential paralysis, occurring in approximately %5 of all patients with myeloma often leads to disability and a profound impact on prognosis, even if myeloma is otherwise contained [5, 11]. MM with spinal involvement may represent with spinal cord or cauda equine compression, with or without neurological impairment. Signs and symptoms of SCC include pain (83–95%), motor defects (60–85%), sensory deficits (40–90%); and bowel and bladder dysfunction. Back pain is typically the first symptom. Weakness is the most apparent and problematic manifestation of SCC. Sensory deficits are less common. Bowel and bladder dysfunction tend to occur late and typically match the degree of weakness [12]. Magnetic resonance imaging (MRI) or computed tomographic (CT) myelography of the entire spine must be performed immediately if this complication is suspected. This review aims to summarize recent development in the treatment of SCC in patients with MM.
Management of spinal cord compression in multiple myeloma
Prompt diagnosis and immediate treatment are critically important in the preservation of neurological function in patients with SCC. Treatment and management of spinal myeloma disease requires a multidisciplinary approach. The goal of the treatment of the SCC is adequate control of pain; relief of spinal cord or cauda equina compression and maintenance of spinal stability; preserving or improving neurologic functions. Management of patients with SCC includes the immediate administration of glucocorticoids in nearly all patients, followed by surgery, external beam radiation therapy (EBRT), or stereotactic body radiotherapy (SBRT). Systemic therapy may be beneficial in patients with chemosensitive tumors. In patients with neurologic symptoms directly due to cord compression, radiation therapy (RT) is given along with dexamethasone, and up to half of patients may have improvement of motor function with RT with longer fractionation schedules providing better relief [13]. Systemic therapy with regimens such as bortezomib, cyclophosphamide, dexamethasone or bortezomib, thalidomide, dexamethasone work rapidly and can be used instead of radiation in selected patients if there is minimal neurologic deficit. Surgical decompression is necessary only if the neurologic deficit does not improve or if the compression is due to retropulsed bone.
Symptomatic treatment
Symptomatic treatment of SCC often begins prior to definitive therapy and consists of the pain management, bed-rest and anticoagulation [14]: Patients with SCC have frequently a severe pain, often limiting the ability to perform a thorough neurologic examination. Corticosteroids usually improve the pain within several hours, but most patients require opiate analgesics to tolerate the physical examination and necessary diagnostic studies. There is generally no need to confine the patient to bed. Patients are generally quite adept at avoiding maneuvers that trigger their pain and there is no risk that movement will worsen the neurologic status. Many patients with cancer are in a hypercoagulable state. Although the value of prophylaxis against venous thromboembolism has not been studied specifically in patients with SCC, anticoagulation (i.e., unfractionated heparin, low molecular weight heparin, fondaparinux) should be considered if the patient is immobilized due to the SCC and there is no active bleeding or other contraindications to the use of anticoagulants [14]. If surgery is planned in the immediate future, anticoagulation should be withheld. If there are contraindications to the use of anticoagulants, such patients should be treated with mechanical prophylaxis, such as pneumatic venous compression devices or graduated compression stockings.
Corticosteroids
The beneficial actions of glucocorticoids in restoring neurologic function in patients with SCC were first reported in the late 1960s. After this, several preclinical and clinical studies investigated the effect of steroids in SCC. Sorensen and colleagues studied the effect of corticosteroids in patients with SCC [15]. The authors randomly assigned 57 patients with carcinoma (two-thirds with a primary in the breast) to receive either dexamethasone (96 mg intravenously followed by 24 mg four times daily for three days and then tapered over 10 days) or no dexamethasone. Their results suggested that a significantly higher percentage of patients in the dexamethasone group remained ambulatory both at the conclusion of therapy (81% versus 63%) and at six months (59% versus 33 %). Significant side effects were seen in three patients (11 percent in the steroid group. In meta-analyses it was concluded that there is insufficient evidence as to the role of corticosteroids and the appropriate initial dose [16].
Corticosteroids relieve pain management, reduce vasogenic cord edema and may prevent additional damage to the spinal cord from decreased perfusion. Therefore high-dose corticosteroid therapy (Initial bolus of 100 mg followed by 96 mg divided into four doses for 3 days) is generally considered to be part of the standard regimen for SCC, despite limited documented evidence of benefit and a significant risk of serious side effects [17]. Several studies have suggested that lower doses can be effective but they have not been assessed in randomized trials. Long-term use is not recommended because of the potential to cause further osteopenia and other steroidrelated adverse effects.
Pain management
Pain is the most common symptom of spinal involvement with myeloma and treatment is aimed at alleviating it as much as possible to preserve quality of life. Patients with SCC are frequently in severe pain, often limiting the ability to perform a thorough neurologic examination. Glucocorticoids usually improve the pain within several hours, but most patients require opiate analgesics to tolerate the physical examination and necessary diagnostic studies. Opioids are the most commonly used analgesics but they may cause significant adverse effects that can reduce quality of life, especially in older patients [18]. Patients with persistent severe pain requiring highdose opioid pain relief should be referred to the palliative pain care specialist [19].
Bisphosphonates
The aim of bisphosphonate treatment is to slow down or prevent the progression of bone destruction and, in the process, can help alleviate bone pain and reduce the risk of skeletal fractures. Zoledronic acid or pamidronate are used for myeloma bone disease. Currently, there is no consensus regarding the optimal duration of bisphosphonate treatment [10]. The monoclonal antireceptor activator of nuclear factor kapa-B ligand (RANKL) antibody denosumab was found to be no inferior to zoledronic acid in preventing or delaying first on study SRE in patients with metastatic to bone or myeloma [20].
Systemic chemotherapy
Patients with a chemosensitive malignancy, chemotherapy are an attractive option because it can also treat tumor deposits elsewhere in the body. Targeting the myeloma cells, anti-myeloma treatments can disrupt the interaction with the bone microenvironment, thus inhibiting the osteoclastogenic effect. Treatment for myeloma is based around various anti-myeloma regimens combining chemotherapy agents with steroids and either proteasome inhibitors or immunomodulatory drugs, with or without high-dose chemotherapy and stem cell transplantation [10, 21]. Systemic therapy with regimens such as bortezomib, cyclophosphamide, dexamethasone (VCD) or bortezomib, thalidomide, dexamethasone (VTD) work rapidly and can be used instead of radiation in selected patients if there is minimal neurologic deficit [14]. Conventional treatment of the myeloma is effective in reducing spinal pain and the risk of further damage. It is not effective in treating spinal fracture pain.
Spinal treatment
The choice of modality for definitive treatment depends on many factors, including the presence of absence of spinal instability, the degree of spinal cord compression, and the relative radio sensitivity of the tumor. An algorithmic approach to treatment based upon these factors is available (Figure 1). An important component of the decision making process when considering definitive therapy is assessment of spinal stability. Treatment of a SCC differs in those patients whose spine is unstable compared to those with a stable spine. Pain from an unstable spine will not be relieved with RT, and there is a lack of evidence on whether spinal bracing is an effective technique for reducing pain [22]. Thus, an unstable spine must be stabilized either by surgery with fixation [23] or by percutaneous vertebral repair [24].
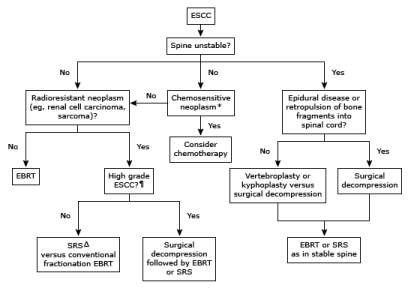
Figure 1: Suggested algorithm for the management of spinal cord compression.
A classification system for spinal instability in neoplastic disease has been developed based upon the available evidence and expert consensus opinion consultation. From the Spine Oncology Study Group consensus opinion was used to derive six individual components of spinal instability, which were scored, with a final Spine Instability Neoplastic Score (SINS) representing a composite score of the individual components (Table 1, 2) According to this classification, patients with a score of 7 or higher are considered to be at risk for spinal instability and warrant surgical consultation [25]. This classification system is not evidence based and it reflects broad expert opinion of spinal neurosurgeons. For some patients, bracing may provide short-term control of pain by stabilizing the spine and reducing the mechanical load on the vertebral bodies. It isn’t generally recommended for any longer than 3 months [10].
Component scores for clinical and radiographic findings
Score
Spine location
Junctional (occiput-C2,C7-T2,T11-L1,L5-S1)
3
Mobile spine (C3-C6,L2-L4)
2
Semi rigid (T3-T10)
1
Rigid (S2-S5)
0
Pain relief with recumbence and/or pain with movement/loading of the spine
Yes
3
No (occasional pain but not mechanical)
1
Pain-free lesion
0
Bone lesion quality
Lytic
2
Mixed lytic/blastic
1
Blastic
0
Radiographic spinal alignment
Subluxation/translation present
4
De novo deformity (kyphosis/scoliosis)
2
Normal alignment
0
Vertebral body collapse
>50 percent collapse
3
< 50 percent collapse
2
No collapse with >50 percent body involved
1
None of the above
0
Posterolateral involvement of spinal elements (facet, pedicle, or costovertebral joint fracture or replacement with tumor)
Bilateral
3
Unilateral
1
None of the above
0
Table 1: Classification system for spine in stability neoplastic score (SINS).
Score
Classification
Action
0-6
Stable spine
7-12
In determinant
Possible impending instability, warrants
Surgical consultation
13-18
Instability
Warrants surgical consultation
Table 2: The SINS score is generated by adding all of the scores from the six individual components (minimal score is 0, maximal score is 18).
Surgery
Surgical management of myeloma spinal disease has been similar to the management of spinal metastases in solid cancers. Initially it was thought that the posterior decompression using a laminectomy for patients with SCC was the initial approach in the patient with neurologic compromise. However, retrospective comparisons of case series of patients treated with laminectomy with or without RT versus RT alone revealed no advantage to the surgical approach [26]. The tumor bulk is usually located in the vertebral body anterior to the cal sac laminectomy may not improve the outcome. Laminectomy alone does not provide sufficient access to resect tumor anterior to the spinal cord and can result in iatrogenic progressive instability. Laminectomy without spine stabilization should be avoided, whenever possible [14]. Anterior reconstruction can be accomplished using either bone grafts or methylmethacrylate (bone cement). Posterior segmental fixation is accomplished using screw rod systems, including pedicle screws in the thoracic and lumbar spine and lateral mass screws in the cervical spine [27].
Patchell and colleagues investigated the benefit of integrating aggressive tumor resection to decompress the spinal cord and spine stabilization into the initial management [27]. This study enrolled 101 patients with metastatic SCC and compared the direct circumferential surgical decompression followed by RT (30 Gy over 10 days, starting within 14 days of surgery) with the same RT alone. Both groups received the same initial dose of glucocorticoids (dexamethasone 100 mg initially, then 24 mg every six hours) and either began RT or underwent surgery within 24 hours of presentation. The planned interim analysis revealed that the patients treated with surgery followed by RT had a significantly higher ambulatory rate (84% versus 57% with to RT alone) and retained the ability to walk significantly longer than those treated with RT alone (median 122 versus 13 days).
Patients with an unstable spine who are not candidates for radical surgery may derive some symptomatic benefit from minimally invasive techniques such as vertebroplasty and kyphoplasty, followed by radiotherapy. Percutaneous vertebroplasty (PV) involves the percutaneous placement of one or two trocars into the vertebral bodies via the pedicles or the extrapedicular approach for the injection of methylmethacrylate under fluoroscopic guidance. Balloon kyphoplasty (BKP) is a modified version of PV and is an alternative treatment for SCC. During BKP, an inflatable bone tamp is passed down the trocar in the same way as during PV, inserted into the fractured vertebra and inflated to create a cavity into which the cement can be injected [10].
In the light of the available data, it can be concluded that aggressive tumor resection and stabilization followed by RT increases the likelihood of regaining the ability to walk and of maintaining ambulation following treatment compared to those treated with RT alone. Careful selection is required to identify those patients with an adequate life expectancy and good medical status who are candidates for this aggressive approach [28]. Although questions have been raised about the benefit of surgery, until further information is available from prospective randomized trials, suitable carefully selected patients should be offered the option of surgical resection. Surgical decompression is the preferred approach for patients with an unstable spine and for relatively radio resistant tumors that compress the spinal cord [14].
Radiation therapy
Approximately 40% of the patients with the diagnosis of MM will require RT to control disease at some point in their disease course [29]. RT is used for patients who are not considered surgical candidates and following surgical decompression. The RT portal covers the width of the vertebral body and all areas of paravertebral tumor extension with margin, is centered on the spine, and typically extends one vertebral body above and below the epidural metastasis. RT is generally very well tolerated. When large segments of the spine are irradiated, bone marrow suppression and gastrointestinal toxicity may complicate treatment [14].
RT may be used as fractionated external beam RT (EBRT) or stereotactic body RT (SBRT). Both of them are effective for palliation and local tumor control. Approximately 70% of patients have an improvement in pain, and one-half of those without spinal instability experience resolution of back pain following EBRT [14,30,31]. However the effectiveness of conventional EBRT is limited by the spinal cord, which is intolerant of high dose RT. On the other hand SBRT utilizes precisely targeted radiation to a tumor while minimizing radiation to adjacent normal tissue. This targeting allows treatment of small- or moderate-sized tumors, even in close proximity to the spinal cord, in either a single or limited number of dose fractions. Although high doses can be delivered, normal tissue constraints are always respected, especially for the spinal cord. This frequently results in tumor adjacent to the spinal cord receiving a lower dose than tumor further away from the spinal cord. Since MM is a relatively radiosensitive tumor both approaches are effective for treatment [30].
EBRT is appropriate definitive therapy for patients who are not considered surgical candidates and who have relatively radiosensitive tumors. It is also indicated after surgical decompression. EBRT may be used either in a single fraction (eg 8 Gy) or protracted courses (eg 40 Gy divided into 20 fractions). Several observational studies have examined the impact of long-course versus shorter-course regimens on functional outcomes, local tumor control, and overall survival. Rades and colleagues compared short-course RT with either 8 Gy in 1 fraction or 20 Gy in 5 fractions with long-course RT with 30-40 Gy in 10-20 fractions [32]. Their results suggested that there were no significant differences in functional outcome (post treatment motor function or the percentage of patients regaining ambulation) or overall survival between the groups. However, long-course EBRT was associated with significantly better local control (77% vs 61%) and 12-month progression free survival (72% vs 55%). In a phase III trial in which 327 patients with SCC and a short life expectancy were randomly assigned to two fractions of 8 Gy (16 Gy total dose) or single dose of 8 Gy, no differences in response or overall survival (median 4 months) were found [33]. In summary, for patients with a relatively short life expectancy, short-course EBRT may be more convenient since it affords similar palliation, without the inconvenience of a more protracted treatment course. Patients with a prolonged natural history, oligometastatic disease without visceral involvement, slow progression of motor deficits, and histologic diagnosis of myeloma may derive benefit from a more protracted course of RT [14].
SBRT is a noninvasive treatment option for spinal disease in the absence of high-grade spinal cord compression. SBRT with a single 24 Gy fraction gives excellent tumor control, even in patients who have relatively radio resistant tumors such as sarcoma and renal cell cancer, and an early diagnosis of SCC before high-grade spinal cord compression has developed. For patients with high-grade SCC who undergo surgical decompression, postoperative high-dose singlefraction or hypofractionated SBRT also appears to provide excellent tumor control [14].
Conclusions
SCC which is an oncologic emergency that can cause pain and potential paralysis, occurring in approximately %5 of all patients with myeloma often leads to disability and a profound impact on prognosis, even if myeloma is otherwise contained. Immediate diagnosis and treatment are critically important in the preservation of neurological function. The main aims of the treatment are pain control, avoidance of complications from local disease progression, and the preservation or improvement of neurologic functioning. An important component of the pretreatment decision making process is an assessment of spinal instability. For patients with SCC and either neurologic symptoms or substantial the cal sac compression by imaging, corticosteroids should be an integral component of the initial management. Surgery and RT either in the form of BRT or SBRT are the primary approaches to treat tumor compressing the spinal cord. Systemic therapy with regimens such as bortezomib, cyclophosphamide, dexamethasone or bortezomib, thalidomide, dexamethasone work rapidly and can be used instead of radiation in selected patients if there is minimal neurologic deficit. Surgical decompression is necessary only if the neurologic deficit does not improve or if the compression is due to retro pulsed bone. Minimally invasive techniques like vertebroplasty and kyphoplasty are only appropriate for patients with symptomatic spinal metastases without significant epidural disease or retropulsion of bone fragments into the spinal cord.
References
- Angtuaco EJ, Fassas AB, Walker R, Sethi R, Barlogie B. Multiple myeloma: clinical review and diagnostic imaging. Radiology. 2004; 231:11-23.
- Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ 3rd. Incidence of multiple myeloma in Olmsted County, Minnesota: trend over 6 decades. Cancer. 2004; 101: 2667-2674.
- Cancer Facts & Figures 2013. Atlanta: American Cancer Society. 2013.
- Greenberg AJ, Vachon CM, Rajkumar SV. Disparities in the prevalence, pathogenesis and progression of monoclonal gammopathy of undetermined significance and multiple myeloma between blacks and whites. Leukemia. 2012; 26: 609-614.
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1,027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78: 21-33.
- Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008; 111: 2521-2526.
- Walker RE, Lawson MA, Buckle CH, Snowden JA, Chantry AD. Myeloma bone disease: pathogenesis, current treatments and future targets. Br Med Bull. 2014; 111: 117-138.
- Coleman RE. Skeletal complications of malignancy. Cancer 1997; 80: 1588-1594.
- Croucher PI, Apperley JF. Bone disease in multiple myeloma. Br J Haematol .1998; 103: 902-910.
- Sean Molloy, Maggie Lai, Guy Pratt, Ramasamy K, Wilson D, Quraishi N, et al. Optimizing the management of patients with spinal myeloma disease. Br J Haematol. 2015; 171: 332-343.
- Basic-kes V, Basic-Jukic N, Kes P, Demarin V, Labar B. Neurologic sequelae of bone changes in multiple myeloma and its therapy. Acta Med Croatica. 2002; 56: 103-107.
- Helweg-Larsen S, Sørensen PS, Kreiner S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000; 45: 1163-1169.
- Rades D, Hoskin PJ, Stalpers LJ, Schulte R, Poortmans P, Veninga T, et al. Short-course radiotherapy is not optimal for spinal cord compression due to myeloma. Int J Radiat Oncol Biol Phys. 2006; 64: 1452-1457.
- Schiff D, Brown P, Shaffrey ME. Treatment and prognosis of neoplastic epidural spinal cord compression, including cauda equina syndrome. Up to date. 2016.
- Sørensen S, Helweg-Larsen S, Mouridsen H, Hansen HH. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomized trial. Eur J Cancer. 1994; 30A: 22-27.
- George R, Jeba J, Ramkumar G, Chacko AG, Leng M, Tharyan P. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev. 2008; CD006716.
- Loblaw DA, Mitera G, Ford M, Laperriere NJ. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys. 2012; 84: 312-317.
- Sloot, S., Boland, J., Snowden, J.A, Ezaydi Y, Foster A, Gethin A, et al. Side-effects of analgesia may significantly reduce quality of life in symptomatic multiple myeloma: a cross-sectional prevalence study. Support Care Cancer. 2015; 23: 671-678.
- Snowden JA, Ahmedzai SM, Aschroft J, D'Sa S, Littlewood T, Low E, et al. Guidelines for supportive care in multiple myeloma. Br J Haematol. 2011; 154: 76-103.
- Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer or multiple myeloma. J Clin Oncol. 2011; 29: 1125-1132.
- Bird JM, Owen RG, D’Sa S, Snowden JA, Pratt G, Ashcroft J, et al. Guidelines for the diagnosis and management of multiple myeloma. Br J Haematol. 2011; 154, 32-75.
- Lee SH, Cox KM, Grant R, Kennedy C, Kilbride L. Patient positioning (mobilisation) and bracing for pain relief and spinal stability in metastatic spinal cord compression in adults. Cochrane Database Syst Rev. 2012; 3: CD007609.
- Wang JC, Boland P, Mitra N, Yamada Y, Lis E, Stubblefield M, et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004; 1: 287-298.
- Mendel E, Bourekas E, Gerszten P, Golan JD. Percutaneous techniques in the treatment of spine tumors: what are the diagnostic and therapeutic indications and outcomes? Spine (Phila Pa 1976). 2009; 34: S93-100.
- Fourney DR, Frangou EM, Ryken TC, Dipaola CP, Shaffrey CI, Berven SH, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol . 2011; 29: 3072-3077.
- Findlay GF. Adverse effects of the management of malignant spinal cord compression. J Neurol Neurosurg Psychiatry. 1984; 47: 761-768.
- Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005; 366: 643-648.
- van den Bent MJ. Surgical resection improves outcome in metastatic epidural spinal cord compression. Lancet. 2005; 366: 609-610.
- Featherstone C, Delaney G, Jacob S, Barton M. Estimating the optimal utilization rates of radiotherapy for hematologic malignancies from a review of the evidence: part II-leukemia and myeloma. Cancer. 2005; 103: 393-401.
- Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radio surgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila Pa 1976). 2009; 34: S78-92.
- Young RF, Post EM, King GA. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg. 1980; 53: 741-748.
- Rades D, Lange M, Veninga T, Rudat V, Bajrovic A, Stalpers LJ, et al. Preliminary results of spinal cord compression recurrence evaluation (score-1) study comparing short-course versus long-course radiotherapy for local control of malignant epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2009; 73: 228-234.
- Maranzano E, Trippa F, Casale M, Costantini S, Lupattelli M, Bellavita R, et al. 8Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol. 2009; 93: 174-179.