
Research Article
Ann Hematol Oncol. 2016; 3(5): 1091.
Tyrosine Kinase Inhibitors Induce Remissions in Patients with Philadelphia-Chromosome Positive Leukemia at Relapse after Allogeneic Stem Cell Transplantation
Wolf A¹, Dreger P¹, Dengler J1,2, Ho AD¹ and Schmitt M¹*
¹Department of Hematology and Internal Medicine V, University Hospital Heidelberg, Germany
²Department of Hematology and Internal Medicine, Oncology Schwerpunktpraxis Heilbronn, Germany
*Corresponding author: Michael Schmitt, Department of Hematology and Internal Medicine V, University Hospital Heidelberg, Heidelberg, Germany
Received: June 08, 2016; Accepted: July 13, 2016; Published: July 15, 2016
Abstract
The advent of tyrosine kinase inhibitors (TKI) for the treatment of Philadelphia-chromosome positive (Ph+) chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) dramatically changed the treatment algorithms for these disease entities. Nevertheless, still some patients with TKI-non-responsive CML as well as high risk-ALL patients with an appropriate donor need to undergo allogeneic stem cell transplantation (allo-SCT). In case of minimal residual disease (MRD) or relapse, treatment with TKI constitutes a therapeutic option to existing forms of relapse treatment such as donor lymphocyte infusion (DLI) or a 2nd allo-SCT. In the current survey we analyzed 17 patients with Ph+ leukemia who relapsed after allo-SCT at our institution from 1998 till 2013. Nine patients suffered from Ph+ CML, eight patients suffered from Ph+ ALL. We demonstrated that the achievement of a complete molecular response (CMR) on day +28 after transplantation is the pivotal prognostic factor for long-time survival after relapse when using TKI. Patients who achieved a CMR had an overall survival of 100% after 5 years. The prognosis for patients, who did not achieve a CMR was dismal: the overall-survival was 0% after 5 years. At the latest follow-up (4-171 months) 11 patients were alive and 7 patients had died. This clear distinction of two prognostic subgroups might be helpful in making decisions towards use of TKIs.
Keywords: Philadelphia chromosome; Chronic myeloid leukemia; Acute lymphoblastic leukemia; Relapse; Tyrosine kinase inhibitor
Abbreviations
a/c GvHD: acute/chronic Graft versus Host Disease; ALL: Acute Lymphoblastic Leukemia; Allo-SCT: Allogeneic Stem Cell Transplantation; AP: Accelerated Phase; BC: Blast Crisis; CCR: Complete Cytogenetic Remission; CHR: Complete Hematologic Response; CML: Chronic Myeloid Leukemia; CMR: Complete Molecular Remission; CP: Chronic Phase; DLI: Donor Lymphocyte Infusion; ELN: European Leukemia Network; IRB International Review Board; MC: Multicenter; MMR: Major Molecular Remission; MR: Molecular Relapse; MRD: Minimal Residual Disease; OS: Overall Survival; PCR: Polymerase Chain Reaction; PD Progressive Disease; PFS Progression-Free Survival; Ph+ Philadelphia-Chromosome Positive; RES: Resistance; SC: Single-Center; TKI: Tyrosine Kinase Inhibitors
Introduction
Chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) are rare malignant diseases with a yearly incidence rate of 1-2 patients per 1, 00, 000 inhabitants in Western countries [1,2].
The advent of the first tyrosine-kinase inhibitor imatinib revolutionized the therapy for Ph+leukemias such as the CML and about 20% of ALL [3].
The use of these drugs in first-, second- or third line therapy resulted in a dramatically decrease in the number of allogeneic stem cell transplantation (allo-SCT) for CML. In 1998 still about 30% of all allogeneic transplanted patients in Germany had CML as diagnosis. In contrast the percentage of allo-SCTs because of CML decreased by factor 10 down to about 3% in 2013[4,5].
However, allo-SCT remains the only curative treatment for CML and Ph+-ALL [6-9]. The main reason for failure of allo-SCT is relapse, occurring in about 35-45%, causing death in about 30-40% of those patients [10]. There are different options for the treatment of CML and ALL relapses after allo-SCT. Reduction of immunosuppression, administration of donor lymphocytes (DLI), eventually the administration of a TKI, or even a second transplantation [10].
Disease stage at relapse is one of the main factors influencing the overall survival of patients [11]. Using real-time-PCR to detect BCR/ABL-transcripts in Ph+ leukemias enables to diagnose an early molecular relapse [12].
In our report, we describe 17 patients who relapsed after allogeneic stem cell transplantation in different disease stages at relapse.
Patients and Methods
In this retrospective, single-center study we analyzed data of 17 patients with Ph+ leukemia who relapsed after allogeneic stem celltransplantation in the period from April 1998 to March 2013 and were treated with TKI consecutive. All patients were treated at the University Clinic Heidelberg. The study followed the Declaration of Helsinki of 1975 and was approved by the International Review Board (IRB) University Clinic Heidelberg. All participants gave informed consent.
Criteria
Inclusion criteria for our study were Ph+ CML or ALL, relapse after allogeneic blood stem cell transplantation, age over 18 years and relapse treatment with tyrosine kinase-inhibitors.
Endpoints of our analysis were remission-status day +28 after transplantation, achievement of complete molecular response with negative PCR for the BCR/ABL transcript and remission status at the latest follow-up.
The overall survival (OS) was measured from the date of relapse to the latest time of follow-up or death.
Collection of data
For our analysis we collected the following data: underlying disease CML or ALL, gender and age at the time of transplantation and relapse, period of time from transplantation to relapse, duration of TKI treatment before allo-SCT, after allo-SCT and after relapse, remission status on day+28, at relapse and at the last follow-up the time span: from allo-SCT to relapse, occurrence of acute or chronic graft-versus-host-disease (GvHD).
We used the “European Leukemia Network (ELN)”criteria for monitoring the response to TKI treatment for CML [13]. Complete hematologic response (CHR) was diagnosed when a normal blood cell count was found and the spleen was not palpable. Complete cytogenetic remission (CCR) was defined by no Ph+ cells in bone marrow. A major molecular remission (MMR) was diagnosed when less than 0.1% transcripts of BCR/ABL were found in peripherical blood. Patients with a complete molecular remission (CMR) had no BCR/ABL transcripts in real time-and nested-PCR and no minimal residual disease (MRD).
We analyzed potential predictors for disease-free survival of patients who relapsed after allo-SCT. Demographics and patients’ characteristics were summarized by descriptive statistics. Survival functions were estimated by using the Kaplan-Meier method.
Results
From 1998 to 2013, 148 patients suffering from CML or ALL underwent an allogeneic stem cell transplantation at the University Clinic Heidelberg. Forty-four of these 148 patients relapsed. Seventeen of these 44 relapsed patients suffered from Ph+ leukemia and were treated with TKI after relapse. Nine patients with CML and eight patients with ALL. The median age at time of transplantation was 39 years and 41 years at the time of relapse. At the last followup 10 patients were alive. Seven patients died within 17 months after relapse. Most (n=10) of the patients relapsed in a chronic phase (CML) or had a molecular response (ALL). Four patients were additionally treated with DLI. Table 1 shows the patient characteristics (Table 1).
Median
Range
Age at Allo-SCT
39 years
19-66 years
Age at relapse
41 years
31-68 years
Follow-up
60 months
4-135 months
IntervalAllo-SCT-relapse
14 months
1-119 months
Intervalrelapse-TKI
1 month
0-9 months
Intervalrelapse-death
9.5 months
2-17 months
Number
Percentage (%)
Gender: male/female
5-Dec
71/29
Last follow-up
11 alive
65
Entity: CML/ALL
8-Sep
53/47
Disease status at relapse
Blast crisis
2
12
PD
5
29
CP/MR
10
59
Previous treatment for relapse
DLI
4
24
Chemotherapy
6
35
Alpha-interferon
2
12
None
4
24
Response to TKI-Treatment
aGvHD
8
47
cGvHD
11
65
CMR
9
53
Table 1: Patients` characteristics.
The estimated median overall survival was 58.8% at 10 years for all 17 patients relapse with a median follow-up of 60 months after relapse.
For patients who had a complete molecular remission (CMR) on day+28 after allo-SCT and relapsed subsequently OS was 100% at 14 years. Patients, who did not reach CMR on day+ 28, had an OS of 0% at 4 years (Figure1).
Figure 2 and Figure 3 shows the treatment process from the date of allo-SCT to the last follow up or death for both entities CML and ALL. Figure 4 shows the abbreviations used in both figures. Patients were divided into two groups a “responder”-group and a “nonresponder”- group, depending on their remission status on day+28 after allo-SCT, as highlighted on the left part of Figure 2 and Figure 3. Treatment relapse is marked with a black star. Additional therapy with DLI is marked with an arrow (Figure 2, 3).
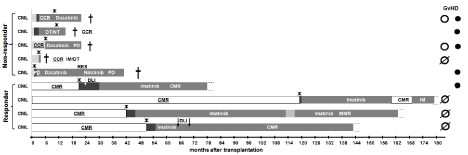
Figure 2: Clinical course of the patients suffering from CML.
The treatment process from the date of allo-SCT to the last follow up or death is shown. Patients were divided into two groups a “responder”-group and a “nonresponder”-
group, depending on their remission status on day+28 after allo-SCT. For explanations of abbreviations see the insert of this figure and the abbreviation
list in the beginning of the manuscript. Crosses indicate the death of the respective patient.
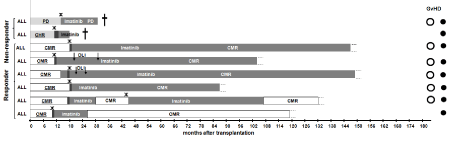
Figure 3: Clinical course of the patients suffering from ALL.
The treatment process from the date of allo-SCT to the last follow up or death is shown. Patients were divided into two groups a “responder”-group and a “nonresponder”-
group, depending on their remission status on day+28 after allo-SCT. For explanations of abbreviations see the insert of this figure and the abbreviation
list in the beginning of the manuscript. Crosses indicate the death of the respective patient.
The timeline of each patient ends up with the last follow-up or death. At the end of each timeline the GvHD-status is illustrated by three signs.
Patients of the responder group had a longer OS than patients from the non-responder group. The duration of molecular remission is much longer than remission of the patients, who did not reach a CMR. The treatment varies from patient to patient. A large difference was observed in the duration of the treatment time with TKI.
The results depicted Figure 1 suggests that the remission status after transplantation is related to the success of relapse treatment. Other factors like the age (<40 years, 40 years), acute GvHD (y/n), chronic GvHD (y/n), DLI treatment (y/n) were not related to a longer survival after relapse and treatment with TKI.
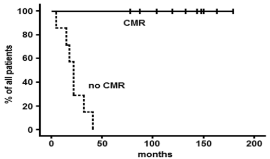
Figure 1: Overall survival according the patient`s status on day +28 after
allo-SCT.
For patients who had a complete molecular remission (CMR) on day+28 after
allo-SCT and relapsed subsequently OS was 100% at 14 years. Patients,
who did not reach CMR on day+ 28, had an OS of 0% at 4 years.
Discussion
Our study supports findings of earlier published reports of relapse treatment after allo-SCT. Ollavaria, et al. showed that treatment with TKI for patients who suffered from CML and relapsed after allo-SCT leads to long-time cytogenetic and molecular remission. They retrospectively analyzed 128 patients. The depth of remission reached before the relapse and the achievement of a hematological response to therapy had a significant effect on the overall survival of the patients. Additional therapy with DLI did result in a better outcome of the patients. The median follow-up was 9 months (2- 34 months) [14]. In 2010 Wright, et al. evaluated the long-term survival of 22 patients with CML who relapsed after allo-SCT. The achievement of a complete molecular remission (CMR) is crucial for a long-term survival and disease control [15]. For patients with Ph+ ALL, Wassmann, et al. showed that patients with detectable BCR/ ABL transcript after allo-SCT reached complete molecular remission (CMR) in about half of the cases after treatment with TKI [16]. Table 2 shows the summarized results of previous discussed studies for relapse treatment with TKI (Table 2).
Author (year)
Type
Patients
Diagnosis/ Phase
Median follow-up after relapse
Treatment
Outcome of all patients
Kantarjian et al. (2002)
SC
28
CML 5 CP, 15 AP, 8 BC
16 months (9-24)
Imatinib
19 patient’s alive, 1-year estimated survival rate: 74%. Overall response rate: 79%
Olavarria et al. (2003)
MC
128
CML 51 CP, 31 AP, 46 BC
9 months (2-34)
Imatinib
86 patients alive, 2-year estimated survival rate : 65%,Overall response rate: 84%
Wassmann et al. (2005)
MC
27
ALL 27 MRD
8.3 months (0.9-31)
Imatinib
27 patients alive, Estimated PFS rate: 48% at 6.6 months (Time to progression)
Wright et al. (2010)
MC
22
CML 8 CP, 14 AP
31.5 months (2-146)
Imatinib (n=20)and/or Dasatinib (n=6)
14 patients alive, 10-year OS from the date of relapse: 54%
Table 2: Previous studies for relapse-treatment with TKI.
Some of our patients have been treated with TKI for a long time after relapse. Treatment safety is an important aspect for a good compliance. Possible side effects of TKIs are: edema, nausea, vomiting, diarrhea and skin rash. Hematological side effects are anemia, thrombocytopenia und neutropenia [17].
Carpenter, et al. showed that prophylactic administration of imatinib at standard dose (400 mg) to high-risk patients with Ph+ CML and ALL at relapse after allo-SCT is rather-well tolerated in terms of side effects of the drug. The most commonly observed side effects were grade 1-3 nausea, vomiting and elevated liver-enzymes [18]. In some cases treatment with TKI might lead to skin-rashes, edema and liver dysfunction [19].
In summary, therapy of patient’s with Ph+ CML or ALL at relapse after allo-SCT seems to be a safe option, when side-effects must are monitored and counterbalanced.
The results of this analysis demonstrate that treatment with TKI after relapse post allo-SCT can lead to a longer survival and that the disease status at the time of relapse is a prognostic factor.
References
- Hoelzer D, Schrappe M and Gökbuget N. Akute Iymphatische Leukämie bei Erwachsenen und Kindern. In: Die Onkologie. Hiddemann W and Bartram CR (eds.) Springer Berlin Heidelberg. 2010; 1672-1702.
- Baccarani M, Dreyling M, Steegmann JL, Muller M, Soverini S, Dreyling M. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 23: 72-77.
- Savage DG, Antman KH. Imatinib mesylate--a new oral targeted therapy. New Engl J Med. 2002; 346: 683-693.
- Deutsches Register für Stammzelltransplantationen (DRST): DRST Jahresbericht. 1998/1999; 22.
- Deutsches Register für Stammzelltransplantationen (DRST): DRST Jahresbericht. 2013; 13.
- Barrett AJ, Horowitz MM, Ash RC, Atkinson K, Gale RP, Goldman JM, et al. Bone marrow transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1992; 79: 3067-3070.
- Snyder DS, Nademanee AP, O'Donnell MR, Parker PM, Stein AS, Margolin K, et al. Long-term follow-up of 23 patients with Philadelphia chromosome-positive acute lymphoblastic leukemia treated with allogeneic bone marrow transplant in first complete remission. Leukemia. 1999; 13: 2053-2058.
- Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004; 22: 4075-4086.
- van Rhee F, Szydlo RM, Hermans J, Devergie A, Frassoni F, Arcese W, et al. Long-term results after allogeneic bone marrow transplantation for chronic myelogenous leukemia in chronic phase: a report from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1997; 20: 553-560.
- Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert Rev Hematol. 2010; 3: 429-441.
- Martinez C, Gomez V, Tomas JF, Parody R, Sureda A, Sanz G, et al. Relapse of chronic myeloid leukemia after allogeneic stem cell transplantation: outcome and prognostic factors: the Chronic Myeloid Leukemia Subcommittee of the GETH (Grupo Espanol de Trasplante Hemopoyetico). Bone Marrow Transplant. 2005; 36: 301-306.
- Baccarani M, Pane F, Saglio G. Monitoring treatment of chronic myeloid leukemia. Haematologica. 2008; 93: 161-169.
- Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European Leukemia Net recommendations for the management of chronic myeloid leukemia. Blood. 2013; 122: 872-884.
- Olavarria E, Ottmann OG, Deininger M, Clark RE, Bandini G, Byrne J, et al. Response to imatinib in patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Leukemia. 2003; 17: 1707-1712.
- Wright MP, Shepherd JD, Barnett MJ, Nantel SH, Sutherland HJ, Tozeet CL, et al. Response to tyrosine kinase inhibitor therapy in patients with chronic myelogenous leukemia relapsing in chronic and advanced phase following allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation. 2010; 16: 639-646.
- Wassmann B, Pfeifer H, Stadler M, Bornhaüser M, Bug G, Scheuring UJ, et al. Early molecular response to post transplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood. 2005; 106: 458-463.
- Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009; 10: 470-481.
- Carpenter PA, Snyder DS, Flowers ME, Sanders JE, Gooley TA, Martin PJ, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007; 109: 2791-2793.
- Kantarjian HM, O'Brien S, Cortes JE, Giralt SA, Rios MB, Shan J, Giles FJ, et al. Imatinib mesylate therapy for relapse after allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2002; 100: 1590-1595.