
Case Report
Ann Hematol Oncol. 2016; 3(5): 1093.
Richter’s Transformation in the Era of Targeted Therapy
Pierce L¹, Lally K², Phan R², Lewis M² and Sharma S³*
¹Department of Internal Medicine, VA Greater Los Angeles Healthcare System, USA
²Department of Pathology, VA Greater Los Angeles Healthcare System, USA
³Department of Hematology Oncology, West Los Angeles VA Medical Center, USA
*Corresponding author: Sanjai Sharma, Hematology Oncology, West Los Angeles VA Medical Center, Los Angeles, California, USA
Received: May 21, 2016; Accepted: July 18, 2016; Published: July 20, 2016
Abstract
Transformation of Chronic lymphocytic leukemia (CLL) into an aggressive lymphoma is described as Richter’s transformation (Richter syndrome, RS). It has a very poor outcome and there are no effective therapies. We describe a case of Richter’s transformation in a CLL patient who had received multiple lines of treatment and was being treated with Bruton’s Tyrosine Kinase inhibitor, Ibrutinib. The CLL continued to respond to Ibrutinib while the patient developed an aggressive clonally related transformation lymphoma.
Keywords: Chronic lymphocytic leukemia; Richter’s transformation; BTK inhibitor
Case Presentation
A 69-year-old man with a 14-year history of chronic lymphocytic leukemia (CLL) was admitted to the medicine service with complaints of shortness of breath, drenching night sweats, and enlarging cervical lymph nodes. The neck nodes were progressively rapidly enlarging and were associated with cough. Computed tomography (CT) scan of neck was performed showing multiple bilateral enlarged cervical lymph nodes with the largest dominant node measuring 5.8 cm. Initial lab findings on admission were significant for a white blood cell (WBC) count of 5700/μL (80% lymphocytes) platelet count 63,000/ μL, hemoglobin 11 gm/dL and LDH of 1109 IU/L (normal 87-165 IU/L). He was a febrile but tachycardic and tachypneic with a chest radiograph suggestive of worsening paratracheal lymphadenopathy and other areas of mediastinal lymphadenopathy as well. He was treated for bacterial pneumonia based on his respiratory culture results and persistent cough. Despite treatment with antibiotics and supportive measures, the patient dyspnea did not improve during the hospital course. A Positron emission tomography (PET-CT) scan demonstrated a high SUV of 13.4 in one of the cervical lymph nodes that was biopsied. The pathology results from his lymph node biopsy were consistent with a diffuse large B-cell lymphoma (DLBCL) with a high proliferation rate (Ki-67 labeling index of 90%), indicative of Richter’s Transformation.
CLL leukemic cells in this patient co-expressed CD5 and CD23 markers, CD38 expression was absent. IgVH was mutated and FISH analysis showed only a 13q14 deletion. The neck lymph node biopsy demonstrated a diffuse proliferation of large cells with ovoid to irregular nuclear contours, vesicular chromatin, one to multiple prominent nucleoli and moderate cytoplasm (Figure 1). Lymphoma cells from the cervical lymph node biopsy were positive for CD20, BCL6, MUM1, BCL2, and CD5 with a high proliferation rate. Interestingly there was loss of CD23 expression in the Richter’s transformation DLBCL while the CLL cells were CD23 positive. Molecular analysis detected identical PCR-amplified dominant DNA bands corresponding to the clonal rearrangements of IGH gene from a lymph node biopsy done three years ago and from the neck node.
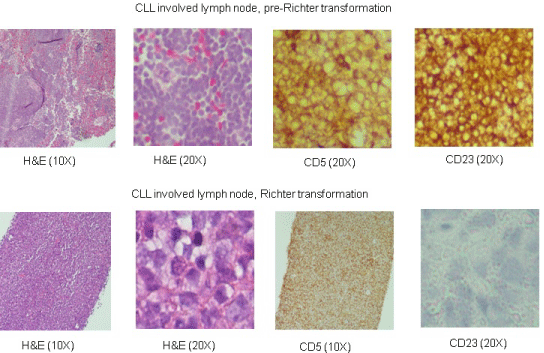
Figure 1: Photomicrographs of CLL involved lymph node (upper panel), three years prior to Richter transformation and from cervical node with Richter’s
transformation (lower panel). Hematoxylin and eosin sections at 10X and 20X immunostaining with CD5 and CD23. Loss of CD23 expression in Richter transformed
tissue (lower panel).
This patient was diagnosed with CLL fourteen years ago and the first chemotherapy regimen was Cytoxan, Vincristine and prednisone (CVP, 3 cycles) four years after the diagnosis. He relapsed a year later and was treated with FCR (Fludarabine, Cytoxan, Rituxan) for four cycles. He relapsed three years later and developed autoimmune hemolytic anemia and was treated with R-CVP (Rituxan–CVP, 2 cycles). Next he was treated with Campath that continued intermittently for two years followed by treatment with Bendamustine and Rituxan (6 cycles). One and half year before the Richter’s transformation Ibrutinib was started which continued till the end. Treatment administered and leukemic cell counts at various stages of the disease are shown in Figure 2.

Figure 2: CLL leukemic cell counts (WBC count) in patient over the course of
illness. Chemotherapy regimens over the years (years since initial diagnosis)
are shown at the bottom. Richter’s transformation was a terminal event.
Treatment options were discussed with the patient but with a poor performance status it was decided not to pursue further chemotherapy. He was discharged home with hospice services and died at home two days after discharge. This case illustrates the progression of this chronic leukemia to one of its worst complications that has a poor prognosis. The case also highlights the need for additional therapeutic options for CLL patients that stop responding to Ibrutinib or transform as they have a remarkably poor prognosis.
Discussion
Richter’s Transformation (Richter’s Syndrome, RS) is an unfortunate complication of CLL that occurs in approximately 2-9% of CLL patients. It is defined by the development of a new aggressive large B-cell lymphoma that can either be clonally related to the patient’s underlying CLL or arise from a de novo mutation. Diagnosis of RS should be considered in CLL patients presenting with elevated serum lactate dehydrogenase (82%), progressive lymphadenopathy (64%), systemic symptoms (59%), monoclonal gammopathy (44%), extranodal involvement (41%) [1]. In a study of 185 patients with untreated CLL, incidence of RS was 9.2% that occurred anywhere from 0 to 82.4 months (6.8 years) after diagnosis, with a median of 23 months [2]. However, as the case above illustrates, it is possible for RT to occur much later as well (14 years in this case). Increasing number of prior therapies is also a suggested risk factor for RS, with one study noting an overall incidence of RT in 5% of CLL patients but as high as 10-13% in the sub-group of patients who had received three or more prior therapies [3]. Risk of development of RS increases when CLL patients are treated with chemotherapy as compared to observation alone and the risk further increases when patients are treated both with alkylating agents and nucleoside analogs [4]. Genetic alterations in RT have been looked at with genome wide DNA microarray profiling and RS specimens were found to have a genomic pattern distinct from CLL and DLBCL. Some of the important genetic alterations were gain of myc, bcl2, mdm4 and losses of p53 and CDKN2A [5, 6]. Notch1 and SF3B1 mutations are known to be associated with poor outcome in CLL and also increase the risk of Richter transformation [7].
RS is associated with a very poor prognosis. Tsimberidou, et al. [8] reviewed data of 3986 patients with CLL/SLL who presented to MD Anderson between 1975 and 2005, 204 patients (5.1%) were found to have had “possible RT” and another 148 patients (3.7%) had biopsyproven RT. The median survival in patients with biopsy-proven RS was 8 months (95% CI, 6 to 10 months). This study identified ECOG performance status > 1 (RR 2.02, p = 0.006), LDH > 1.5x upper limit of normal (RR 1.82, p = 0.003), platelet count < 100,000/μL (RR 1.69, p = 0.012), tumor size > 5 cm (RR 1.61, p = 0.022), and more than one prior therapy (RR = 1.62, p = 0.024) as poor prognostic factors.
A number of salvage therapies have been tried in RS patients but the response rates are low. In the 2006 study done at M.D. Anderson, the overall response rate of RS patients treated with chemotherapy was 39% [8]. 12-14% had complete remission and 25% had a partial remission. 20 patients subsequently underwent Stem Cell Transplantation (SCT), 7 patients were in at least partial remission and 13 were treated as salvage therapy for relapsed or refractory RS. Cumulative 3-year-survival was 75% in those who underwent SCT after remission compared 21% of patients who underwent SCT for relapsed/refractory RS. A number of chemotherapy regimens have been tried, including, R-CHOP, Hyper CVXD, OFAR, Bendamustine and Ibrutinib, a BTK (Bruton’s tyrosine kinase) inhibitor [9].
Interestingly, this patient was on Ibrutinib when he developed RS and the CLL was responding very well with steadily decreasing WBC count for one and a half year (last WBC count, 5000/μL). This novel therapeutic agent is very effective with a high response rate of 84% in previously untreated patients and 42.6% in relapsed refractory CLL patients [10,11]. With a median follow up of 18 months no disease progression was observed in previously untreated patients who received Ibrutinib [10]. However both Richter’s transformation and progressive disease are observed with Ibrutinib in previously extensively treated CLL patients. The combined incidence of progressive disease and RS in two studies of Ibrutinib treated patients (average of 3 prior chemotherapy regimens) is in the range of 10%-13% [12,13]. A recent study is now reporting that around 30% of patients discontinue Ibrutinib for various reasons including progressive disease, Richter’s transformation, infections and stem cell transplants. These patients who discontinue Ibrutinib often do very poorly with a reported average survival of only 3 months [12]. This cohort of CLL patients is evidently heavily pre-treated with an average of three different chemotherapy regimens however it appears that survival of these Ibrutinib treated patients is worse than survival of previously reported RS patients in older studies. This difference in survival could be a function of exposure to increasing number of different chemotherapeutic agents that are now being used effectively to manage this leukemia for a long time that in turn leads to a number of treatments induced genetic alterations. As more patients receive Ibrutinib as first line therapy the overall prognosis and survival of this cohort of CLL patients after discontinuing Ibrutinib will be interesting to follow in the future.
In our patient, the transformed lymphoma B cells showed the identical immunoglobulin heavy chain rearrangement as the CLL (clonally related) but certainly acquired additional genetic alterations to transform to DLBCL and loose Ibrutinib sensitivity. Clonally related transformations are known to have a poorer prognosis as compared to the clonally un-related transformations. These Richter transformed lymphoma cells were not sensitive to BTK inhibition as this was a rapidly growing lymphoma while CLL leukemic cells in the peripheral blood continued to respond as indicated by low WBC counts.
References
- Robertson LE, Pugh W, O'brien S, Kantarjian H, Hirsch-Ginsberg C, Cork A, et al. Richter's syndrome: a report on 39 patients. J ClinOncol. 1993; 11: 1985-1989.
- Rossi D, Cerri M, Capello D, Deambrogi C, Rossi FM, Zucchetto A, et al. Biological and clinical risk factors of chronic lymphocytic leukaemia transformation to Richter syndrome. Br J Haematol. 2008; 142: 202-215.
- Tsimberidou AM, Keating MJ. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer. 2005; 103: 216-228.
- Parikh SA, Rabe KG, Call TG, Zent CS, Habermann TM, Ding W, et al. Diffuse Large B-Cell Lymphoma (Richter Syndrome) in Patients with Chronic Lymphocytic Leukaemia: A Cohort Study of Newly Diagnosed Patients. Br J Haematol. 2013; 162: 774-782.
- Rossi D, Spina V, Deambrogi C, Rasi S, Laurenti L, Stamatopoulos K, et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood 2011; 3391-3401.
- Rossi D, Rasi S, Spina V, Fangazio M, Monti S, Greco M, et al. Different impact of NOTCH1 and SF3B1 mutations on the risk of chronic lymphocytic leukemia transformation to Richter syndrome. Br J Haematol. 2012; 158: 426-429.
- Chigrinova E, Rinaldi A, Kwee I, Rossi D, Rancoita PM, Strefford JC, et al. Two main genetic pathways lead to the transformation of chronic lymphocytic leukemia to Richter syndrome. Blood. 2013; 122: 2673-2682.
- Tsimberidou AM, O'brien S, Khouri I, Giles FJ, Kantarjian HM, Champlin R, et al. Clinical outcomes and prognostic factors in patients with Richter's syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J ClinOncol. 2006; 24: 2343-2351.
- Parikh SA, Kay NE, Shanafelt TD. How we treat Richter syndrome. Blood. 2014; 123: 1647-1657.
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015; 373: 2425-2437.
- Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. RESONATE Investigators. N Engl J Med. 2014; 371: 213-223.
- Jain P, Keating M, Wierda W, Estrov Z, Ferrajoli A, Jain N, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015; 125: 2062-2067.
- Maddocks KJ, Ruppert AS, Lozanski G, Heerema NA, Zhao W, Abruzzo L, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients with Chronic Lymphocytic Leukemia. JAMA Oncol. 2015; 80-87.