
Research Article
Ann Hematol Oncol. 2016; 3(5): 1094.
Epstein–Barr Virus Status and Its Prognostic Value in Lymphoma
Wang BJ, Cen XN*, Ren HY*, Liu W, Liang ZY, Qiu ZX, Ou JP, Wang WS, Li Y, Dong YJ, Wang MJ, Wang LH and Wang Q
Department of Hematology, Peking University First Hospital, China
*Corresponding author: Xi-Nan Cen, Department of Hematology, Peking University First Hospital, No.8 Xi Shi ku Street, Xi Cheng District, Beijing, China
Han-Yun Ren, Department of Hematology, Peking University First Hospital, No.8 Xi Shi Ku Street, Xi Cheng District, Beijing, China
Received: June 14, 2016; Accepted: July 25, 2016; Published: July 27, 2016
Abstract
Aim: EBV status is closely associated with the oncogenesis and clinical course of lymphoma, but the significance of EBV status discords. We conducted our study to investigate the EBV status among different types of lymphoma and evaluate the prognostic value of different detections of EBV.
Methods: 263 lymphoma patients who received EBER-ISH, LMP-1 staining or peripheral blood EBV-DNA quantification were recruited. EBV status was demonstrated according to different lymphoma types and the correlations were analyzed between different detections of EBV status and lymphoma clinical courses.
Results: EBV status varies among different types of lymphoma, in which Extra nodal NK/T cell lymphoma, nasal type and angioimmunoblastic T cell lymphoma had relatively high EBER and EBV-DNA positive rate while diffuse large B cell lymphoma(unspecified) and follicular lymphoma often only has concomitant EBV virosis. EBV-DNA positive in EBER/LMP-1 positive lymphoma was associated with worse IPI scores (P=0.030) and initial treatment outcome (P=0.027), furthermore, the EBV-DNA positive did markedly influence OS (P=0.005) and PFS (P=0.003). With regard to the different subtypes, EBER/ LMP-1 positive did not make differences to the OS and PFS of T-NHL, B-NHL and HL. Whereas, T-NHL and B-NHL with positive EBV-DNA demonstrated substantially poorer OS and PFS.
Conclusion: Although EBV status varies among different lymphoma types, positivity of peripheral blood EBV-DNA is often associated with the presence of B symptoms, elevated LDH level and high risk IPI scores. These patients are likely to pursue a more deteriorating clinical course with poorer treatment response, OS and PFS.
Keywords: Lymphoma; Epstein - Barr virus status; EBV-DNA; EBER; LMP- 1; Prognosis
Introduction
Since it was first identified in 1964, the Epstein–Barr virus (EBV) has been a very successful member of the herpes virus family and it is the most common virus closely associated with human lymphoma [1]. About 90-95% adults have been infected by EBV and majority of these people become persistent carriers as a result of latent infection. In which, about 10% resting B lymphocytes will transform into permanent and activated lymphoblastoid cell lines which are mostly mediated by the restricted expression of EBV-encoded latent genes such as latent membranous protein 1(LMP-1) [2,3].
EBV status differs in different types of lymphoma. Up till now, several lymphoma types, such as extra nodal NK/T-cell lymphoma nasal type, angioimmunoblastic T-cell lymphoma, have been recognized as EBV-associated lymphoma because of the close correlation with EBV infection. However, the oncogenesis of some lymphoma types is weakly associated with EBV status, such as Mantle lymphoma, Follicular lymphoma and etc. In addition, EBV status of certain lymphoma type has regional and racial differences.
In the meantime, EBV status is recognized to be a prognostic biomark for the clinical course of lymphoma but the best detection of EBV status is still uncertain. Dupuis J, et al. reported that EBVencoded small nuclear RNAs (EBER) was associated with a worse overall survival (OS) in elderly population of nodal peripheral T-cell lymphoma, unspecified [4]. As to Extra nodal NK/T cell lymphoma, nasal type, detectable plasma EBV-DNA was found to be associated with clinical characteristics and it served as a good indicator for the OS rate [5]. Furthermore, the OS rate of Hodgkin’s lymphoma patients was shown to be closely linked to positive EBER /LMP or plasma EBV-DNA [6-8].
Given that EBV status differs among different lymphoma types, regions and the prognostic value are not well recognized, the EBV status of lymphoma cases should be identified for large population size and the prognostic value of different types of EBV detections should be analyzed and verified by more studies. Therefore, we conducted our study to gain a better insight of the EBV significance in lymphoma.
Materials and Methods
Patients selection
The criteria for case inclusion were as follows: (1) Pathologically confirmed diagnosis of lymphoma according to the World Health Organization classification [9]. (2) Detection of EBV status was available including EBER in situ/LMP-1 and/or peripheral blood EBV-DNA quantification. (3) No prior history of immunodeficiency diseases including AIDS. (4) No prior history of chemotherapy or radiotherapy. The research was in compliance of the Declaration of Helsinki and approved by the ethics committee of the Peking University First Hospital. Written informed consent was obtained from each adult participant. As for the children, written informed consents were obtained from their guardians on behalf of them.
Materials
A complete set of clinical information in this study included the following: patient demographics, lymphoma type, tumor stage and group according to Ann-Arbor stage [10], International Prognostic Index (IPI) score [11], treatment outcome and vital status. Treatment outcome evaluated by repeated CT or PET/CT was defined as complete response (CR), partial response (PR), stable disease (SD), relapsed disease (RD) or progressive disease (PD) according to the revised response criteria [12].
EBV-encoded small nuclear RNAs (EBER) in situ hybridization and/or immunohistochemistry for latent membrane protein 1(LMP- 1) was done to identify EBV-tissue status. EBER-1 oligonucleotide probes were provided by Zhong Shan Biological Technology Company of China and were operated in accordance with the instruction. A known case of EBV-positive post transplant lymphoproliferative disorder was used as a positive control and the sense RNA probes served as negative control. Nuclear staining was interpreted as EBER expression. A positive reaction was defined as more than 5% nuclear positive of examined cells. The anti-LMP IgG1 (Dako, Glostrup, Denmark) was applied to detect the presence of LMP-1. The staining of tumor cells was defined as positive reactivity.
Peripheral blood sample prior to treatment and/or during treatment was collected into an EDTA-containing tube and centrifuged to isolate mononuclear cells and plasma using a Ficoll- Hypaque gradient method for EBV-DNA quantification. The EBV polymerase chain reaction fluorescence quantitative commercial kit was provided by Da An Gene Company of SUN YAT-SEN University and performed by skilled lab technician in accordance with the instruction. The EBV-DNA greater than 500 copies/ml was regarded as EBV-DNA positive.
Statistical analysis
Patient characteristics and remission rates were compared using the chi-squared test or Fisher exact test, as appropriate. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause or the last follow-up. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of first progression or relapse, death from any cause or the last follow-up visit. Patient survival data were analyzed with the method of Kaplan- Meier and compared using the log-rank test. Data were analyzed with SPSS13, P values less than 0.05 were considered statistically significant and all P values correspond to 2-sided significance tests.
Results
In total, 263 cases dated from January 2008 to April 2014 have been included in the analysis. The age of the patients ranged from 5 to 91 years old with the median age around 54 years old. The ratio between male and female patients is 160/103. All the patients were Chinese yellow people. There were 135 B-NHL cases, 94 T-NHL cases and 34 Hodgkin’s lymphoma cases.
Correlation between different types of detections of EBV
In summary, 100 cases received EBER-ISH test, 65 cases received LMP-1 test and 4 received both. Among these, 70 cases were identified to be EBER positive and 14 cases were LMP-1 positive. However, there were two cases with discordant EBER and LMP-1 stains but both of these were considered to be positive (EBER/LMP-1 positive). Peripheral blood mononuclear cells (PBMCs) EBV-DNA quantification assay was conducted for 233 cases, of which 104 cases tested plasma EBV-DNA at the same time. We defined either plasma or PBMC’s EBV-DNA positive as EBV-DNA positive (peripheral blood EBV-DNA positive). The results showed 90 cases were EBV positive, furthermore, 24 cases among them were both plasma and PBMC’s EBV positive. Significant correlation was observed between PBMCs with plasma EBV-DNA positive (r=0.408, P=0.000) and EBER/LMP-1 positive with PBMCs EBV-DNA positive (r=0.223, p=0.008).
EBV status among different types of lymphoma
We evaluated the EBV status by EBER-ISH, LMP-1 and peripheral blood EBV-DNA according to pathological type (Table 1). Extra nodal NK/T cell lymphoma, nasal type had the highest EBERISH positive rate of 97.4% and the peripheral blood EBV-DNA had a positive rate of 74.3%. The positive rate of EBER and EBV-DNA in angioimmunoblastic T cell lymphoma cases was relatively high, which is 82.3% and 56.3%, respectively. In total, 12 out of 13 peripheral T cell lymphoma, unspecified cases were available for EBER-ISH and the positive rate was 33.3%, which was lower than the positive rate of EBV-DNA (54.5%). However, other T cell lymphoma such as T Lymphoblastic cell lymphoma, anaplastic large cell lymphoma and hepatosplenic T cell lymphoma had a relatively low EBV positive rate. Both of the two EBV-associated DLBCL cases had positive peripheral blood EBV-DNA. However, other DLBCL had a lower EBV-DNA positive rate of 19.1%.Both of the 2 cases of grey zone lymohoma (DLBCL/Burkitt’s) had positive EBER staining and peripheral blood EBV-DNA. The positive rate of peripheral EBV-DNA in Follicular lymphoma was 25% while the rate of mantle cell lymphoma was 75%. Among the 34 Hodgkin’s lymphoma cases, 8 cases conducted EBER staining and 7 of them were positive, 11 out of 16 LMP-1 staining cases were positive. Furthermore, the positive rate of peripheral blood EBV-DNA was almost 35%.
Type of lymphoma
case
EBER Positive
LMP-1 Positive
Peripheral EBV-DNA Positive
Extranodal NK/T celllymphoma,
38
97.40%
NA2
74.30%
nasal type
(37/38)
(26/35)
Angioimmunoblastic
18
82.30%
50%
56.30%
T-cell lymphoma
(14/17)
(1/2)
(9/16)
Peripheral T-cell
13
33.30%
0
54.50%
Lymphoma(unspecified)
(4/12)
(0/1)
(6/11)
Hepatosplenic T cell
3
0
NA
33.30%
Lymphoma
(0/3)
(1/3)
Enteropathy-associated
3
66.70%
NA
100%
T cell lymphoma
(2/3)
(2/2)
Cutaneous T cell
2
100%
NA
50%
Lymphoma
(1/1)
(1/2)
T Lymphoblastic cell
8
NA
NA
0
lymphoma
(0/8)
Anaplastic large cell
9
0
0
44.40%
lymphoma
(0/2)
(0/2)
(4/9)
Diffuse large B-cell
96
0
5.90%
19.10%
lymphoma (DLBCL)
(0/9)
(2/34)
(17/89)
EBV-positive DLBCL
2
100%
NA
100%
of the elderly
(2/2)
(2/2)
Grey zone lymphoma
2
100%
0
100%
(DLBCL/Burkitt’s)
(2/2)
(0/1)
(2/2)
Follicular lymphoma
8
0
0
25%
(0/0)
(0/0)
(2/8)
Mantle cell lymphoma
8
0
0
75%
Other B-cell lymphoma1
19
14.30%
0
40%
(1/7)
(0/1)
(6/14)
Hodgkin’s lymphoma
34
87.50%
40.70%
34.80%
(7/8)
(11/16)
(8/23)
- Other B-cell lymphoma included 7 cases of marginal zone cell lymphoma, 5 cases of other small lymphocytic lymphoma, 2 cases of plasmablastic cell lymphoma, 3 cases of Lymphoplasmacytic lymphoma, 1 case of DLBCL/HL.
- NA – Not acquired.
Table 1: The pathological type and EBV status of the 263 cases.
Impact of peripheral blood EBV-DNA positive on survival of EBER/LMP-1 positive lymphoma
In total, there were 50 EBV-DNA positive cases and 21 EBVDNA negative cases (Table 2). Compared with peripheral blood EBV-DNA negative group, the EBV-DNA positive group did not show significant differences in patient gender, age, tumor stage/ group. However, the EBV-DNA positive group had worse IPI scores (P=0.030) and initial treatment outcome (P=0.027). During the follow-up, 3 EBV-DNA negative cases died, the 3-year OS and PFS were 88.2% and 76.7%, respectively. The EBV-DNA positive group showed an obvious deteriorating clinical course with a median PFS of only 9 months (95%CI, 4.03-13.97). The 3-year OS and PFS were 49.7% and 32.3%, respectively. There were significant differences between the two groups as shown in Figure 1. For the 27 cases who received EBV-DNA analysis both in PBMCs and in plasma, 13 cases were found both plasma and PBMC EBV-DNA positive, 14 cases were only PBMC EBV-DNA positive. The clinical course of the 13 cases with both plasma and PBMC EBV-DNA positive were obviously worse whose median survival time was 8 months (P=0.020).
EBV-DNA(+) group
EBV-DNA(-) group
p-value
No (%)
No (%)
Cases
21
Age
0.901
46y or younger
27(54.0)
11(52.4)
older than 46y
23(46.0)
10(47.6)
Gender
0.656
Male
33(66.0)
15(71.4)
Female
17(34.0)
6(28.6)
Type of lymphoma
0.053
B-NHL
5(10.0)
2(10.0)
T-NHL
40(80.0)
12(57.1)
HL
5 (10.0)
7 (32.9)
Stage
0.599
?-?
14(28.0)
7(33.3)
?-?
36(72.0)
14(66.7)
B symptom
Yes
13(26.0)
8(38.1)
0.308
No
37(74.0)
13(61.9)
IPI score
Low-risk (0-1)
10(20.0)
8(38.1)
0.03
Intermediate-risk (2-3)
28(56.0)
13(61.9)
High-risk (=5)
12(24.0)
0(0)
Treatment response
CR
14(28.6)
10(55.6)
0.027
PR
6(12.2)
4 (22.2)
PD/R
29(59.2)
4(22.2)
Table 2: Peripheral EBV-DNA positive group and negative group in EBER/LMP-1 positive patients.
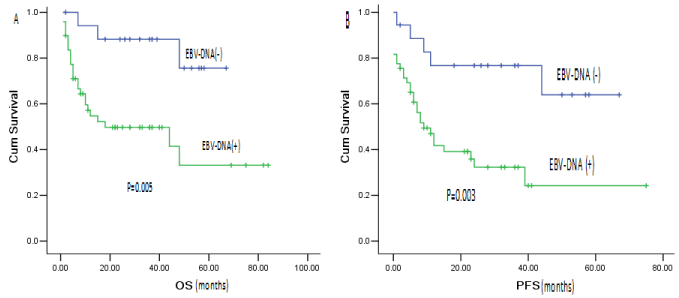
Figure 1: The impact of peripheral blood EBV-DNA positive on overall survival and progression-free survival of EBER/LMP-1 positive patients.
(A) OS by EBV-DNA status in EBER/LMP-1 positive cases. (B) PFS by EBV-DNA status in EBER/LMP-1 positive cases.
Prognostic value of different detections of EBV for lymphoma subtype
Details of the 94 T-NHL cases were demonstrated in Table 1. Among this group, 78 patients were received EBER/LMP-1 staining, in which 58 cases were EBER/LMP-1 positive. At the same time, peripheral blood EBV-DNA tests of 86 patients were done and 49 patients were EBV positive. Comparing with EBER/LMP-1 positive group and negative group, there was no significant difference of the OS (EBER/LMP-1+ vs EBER/LMP-1-; median 44months vs 29 months; P=0.953) and PFS (EBER/LMP-1+ vs EBER/LMP-1-; median 12 months vs 24 months; P=0.961). However, the EBV-DNA positive cases demonstrated substantially poorer OS (EBV-DNA+ vs EBV-DNA-; median 12 months vs 139 months; P=0.005) and PFS (EBV-DNA+ vs EBV-DNA-; median 7 months vs 56 months; P=0.002) (Figure 2A,B).
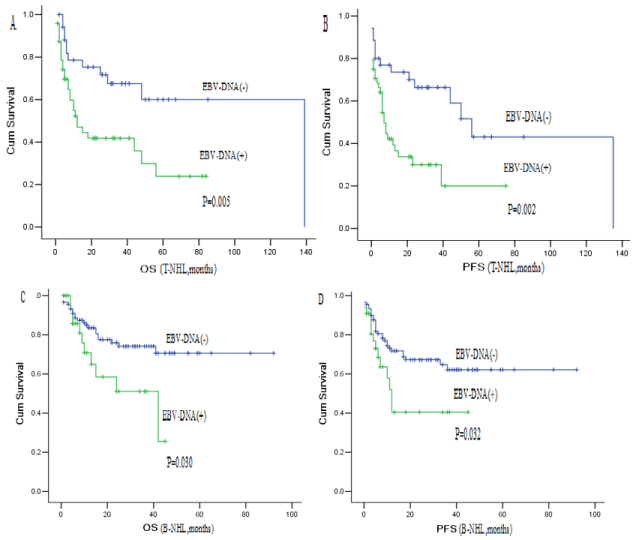
Figure 2: Overall survival and progression-free survival according to peripheral blood EBV-DNA of T-NHL and B-NHL.
(A) OS by peripheral blood EBV-DNA status of T-NHL. (B) PFS by peripheral blood EBV-DNA status of T-NHL. (C) OS by peripheral blood EBV-DNA status of
B-NHL. (D) PFS by peripheral blood EBV-DNA status of B-NHL.
135 B-NHL cases were included in this study. Details were shown in table 1. In which, 56 cases had EBER/LMP-1 staining; 7cases were EBV tissue-positive. For peripheral EBV-DNA tests, 33 of 124 cases were EBV-DNA positive. Comparing EBER/LMP-1 positive group and negative group, there were not significant differences of OS (P=0.817) and PFS (P=0.832), respectively. However, the EBV-DNA positive obviously worsen OS (EBV-DNA+ vs EBV-DNA-: median 42months vs not reached; P=0.030) and PFS (EBV-DNA+ vs EBVDNA-; median 12 months vs not reached; P=0.032) (Figure 2C,D).
In addition, there were 34 Hodgkin’s lymphoma cases in this study. 31 patients had EBER/LMP-1 staining and 15 cases showed EBV tissue-positive. 23 patients had peripheral blood EBV-DNA positive and 8 cases were EBV-DNA positive. The 3-year OS and PFS were 89.9% and 61.3%, respectively. There were not obvious differences of OS (P=0.403) and PFS (P=0.296) between EBER/LMP- 1 positive and negative group. Furthermore, OS and PFS of EBVDNA positive group did not show obvious difference from the EBVDNA negative group (OS: P=0.500, PFS: P=0.700).
Discussion
Detection of EBV infection can be achieved by various methods. EBER can be expressed in all three latency programs and is regarded as the golden standard. LMP-1 is recognized to be closely associated with the clinical course of lymphoma [13]. Therefore, we chose EBER and LMP-1 as the pathological marker of EBV status in this study. Quantification of EBV-DNA in peripheral blood has been demonstrated to be useful for diagnosis and monitoring of EBVassociated diseases. However, whether the PBMCs or plasma is the optimal sample remained uncertain [6,14]. In our study, significant correlation was observed between PBMC and plasma EBV-DNA copies (r=0.408, P=0.000), paralleling the results of previous literature [6]. On that account, we defined either plasma EBV-DNA or PBMCs EBV-DNA positive as EBV-DNA positive in this study.
With regard to EBV status of lymphoma, we described the EBER/ LMP-1 and peripheral blood EBV-DNA status of types of lymphoma in this study (Table 1). As the previous literature showed, our results also verified the close correlation between EBV and certain kinds of lymphoma, such as extra nodal NK/T cell lymphoma, nasal type, angioimmunoblastic T-cell lymphoma, elderly EBV-associated DLBCL and Hodgkin’s lymphoma [15-17]. Furthermore, our study demonstrated the EBV status of mantle lymphoma, follicular lymphoma and other B cell lymphoma, which showed a relatively high EBV-DNA positive rate. We believe, the concomitant EBV virosis cannot be ignored and needs further explore.
The correlations between clinical course and EBV status were investigated by many studies [4-8]. More and more attention was paid to the EBV-targeted immunotherapy to improve these EBVassociated lymphoma outcome and long-term survival condition [18-20].
In our study, firstly, we focused on the EBV-associated lymphoma cases. Compared with peripheral blood EBV-DNA negative group, peripheral blood EBV-DNA positive group had worse IPI scores (P=0.030) and initial treatment outcome (P=0.027). Furthermore, the OS and PFS of the EBV-DNA positive group demonstrated an obvious deteriorating clinical course. The hypothesis that the plasma EBV-DNA is due to tumor release of EBV fragments and its viral load is influenced by tumor load gets already accepted, which can also be explained by our conclusion [9,21]. However, there were some patients with positive PBMC’s EBV-DNA and negative plasma EBVDNA who showed a worse clinical course in this study. We regarded that elevated PBMC’s EBV-DNA may be due to impaired immune surveillance and this leads to bad treatment response and more treatment complication associated with prognosis [22].
Given our series comprised lymphomas of various histologic subtypes and the prognosis were heterogeneous; we divided all the cases to three groups to analyze the correlation with EBV status and clinical courses. In terms of T-NHL and B-NHL, peripheral blood EBV-DNA positive rather than EBER/LM-P-1 positive predicted a worse clinical course. Previous literature also referred that EBER positive in specimen may reflect the replication of EBV [6], but a raised level of circulating EBV-DNA is a risk factor in developing EBV-positive lymphoma [22]. Furthermore, impaired immune surveillance leads to elevated PBMC EBV-DNA, which also brings on worse outcome. With regard to Hodgkin’s lymphoma, however, EBER/LMP-1 and peripheral blood EBV-DNA positive were not closely linked with OS and PFS of the cases in our study, which disagreed with the previous studies [6-8,23]. It may be the result of the limited cases of our study and needs to be explored in further study.
Conclusions
EBV status varies among different lymphoma types. Extra nodal NK/T cell lymphoma, nasal type and angioimmunoblastic T cell lymphoma had relatively high EBER and EBV-DNA positive rate but B cell lymphoma such as mantle lymphoma often has concomitant EBV virosis. With regard to EBER/LMP-1 positive lymphoma, peripheral blood EBV-DNA positive was linked with higher IPI scores, poorer initial treatment outcome and worse overall survival and progressionfree survival. Furthermore, the cases whose PBMNCs and plasma EBV-DNA were both positive demonstrated the worst survival condition. In terms of all T-NHL and B-NHL, peripheral blood EBVDNA positive also predicts a worse OS and PFS. Thus, more attention should be paid to the EBV status of lymphoma and more effective treatment may be adopted in these EBV-positive patients in addition to conventional chemo-therapy.
Acknowledgements
We thank Dr. Lin Nong of pathology department, Peking University First Hospital for helpful identification of pathological files. This study is supported by a grant of the National Natural Science Fund of China (No.81041002), the key topics fund of Beijing Municipal Science and Technology Commission (No. Z141107002514017), project for young teacher of Ministry of Education (No. 20110001120052) and the National Natural Science Fund for the young (No.81200404).
References
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet.1964; 1: 702-703.
- Pope JH, Horne MK, Scott W. Transformation of foetal human leukocytes in vitro by filtrates of a human leukemic cell line containing herpes-like virus. Int J Cancer.1968; 3: 857-866.
- Pattengale PK, Smith RW, Gerber P. Selective transformation of B lymphocytes by E.B. virus. Lancet. 1973; 2: 93-94.
- Dupuis J, Emile JF, Mounier N, Gisselbrecht C, Martin-Garcia N, and Petrella T, et al. Prognostic significance of Epstein – Barr virus in nodal peripheral T–cell lymphoma, unspecified: A Groupe d'Etude des Lymphomes de l'Adulte (GELA) study. Blood. 2006; 108: 4163–4169.
- Suzuki R, Yamaguchi M, Izutsu K, Yamamoto G, Takada K, Harabuchi Y, et al. Prospective measurement of Epstein–Barr virus–DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T–cell lymphoma, nasal type. Blood. 2011; 118: 6018–6022.
- Jarrett RF, Stark GL, White J, Angus B, Alexander FE, Krajewski AS, et al. Impact of tumor Epstein–Barr virus status on presenting features and outcome in age-defined subgroups of patients with classic Hodgkin lymphoma: a population-based study. Blood. 2005; 106: 2444-2451.
- Diepstra A, van Imhoff GW, Schaapveld M, Karim-Kos H, van den Berg A, Vellenga E, et al. Latent Epstein-Barr virus infection of tumor cells in classical Hodgkin’s lymphoma predicts adverse outcome in older adult patients. J Clin Oncol. 2009; 27: 3815-3821.
- Gandhi MK, Lambley E, Burrows J, Dua U, Elliott S, Shaw PJ, et al. Plasma Epstein-Barr Virus (EBV) DNA is a biomarker for EBV-positive Hodgkin's Lymphoma. Clin Cancer Res. 2006; 12: 460-464.
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, et al. World Health Organization Classification of Tumors of the Haematopoietic and Lymphoid Tissues. IARC Press: Lyon. 2008.
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswold’s meeting. J Clin Onc. 1989; 7: 1630-1636.
- A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993; 329: 987-994.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J of Clin Oncol. 2007; 25: 579-586.
- Mao Y, Lu MP, Lin H, Zhang da W, Liu Y, Li QD, et al. Prognostic Significance of EBV Latent Membrane Protein1 Expression in Lymphomas: Evidence from 15 Studies. PLoS One. 2013: 8: e60313.
- Chan KC, Zhang J, Chan AT, Lei KI, Leung SF, Chan LY, et al. Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res. 2003: 63: 2028-2032.
- Gualco G, Domeny-Duarte P, Chioato L, Barber G, Natkunam Y, Bacchi CE, et al. Clinicopathologic and molecular features of 122 Brazilian cases of nodal and extranodal NK/T-cell lymphoma, nasal type, with EBV sub typing analysis. Am J Surg Pathol. 2011: 35: 1195-1203.
- Anagnostopoulos I, Hummel M, Finn T, Tiemann M, Korbjuhn P, Dimmler C, et al. Heterogeneous Epstein-Barr virus infection patterns in peripheral T-cell lymphoma of angioimmunoblastic lymphadenopathy type. Blood. 1992: 80: 1804-1812.
- Williams H, Crawford DH. Epstein-Barr virus: the impact of scientific advances on clinical practice. Blood. 2006; 107: 862-869.
- Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N EnglJ Med 1994: 331: 679-680.
- Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV + lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012; 119: 2644-2656.
- Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014; 32: 798-808.
- Au WY, Pang A, Choy C, Chim CS, Kwong YL. Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood. 2004; 104: 243-249.
- Khan G, Lake A, Shield L, Freeland J, Andrew L, Alexander FE, et al. Phenotype and frequency of Epstein-Barr virus-infected cells in pretreatment blood samples from patients with Hodgkin lymphoma. Br J Haematol. 2005; 129: 511-519.
- Kanakry JA, Li H, Gellert LL, Lemas MV, Hsieh WS, Hong F, et al. Plasma Epstein-Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: correlative analysis from a large North American cooperative group trial. Blood. 2013; 121: 3547-3553.