
Research Article
Ann Hematol Oncol. 2016; 3(6): 1099.
The Presence or Absence of Barrett’s Esophagus Affects the Prognosis of Patients with Esophageal Adenocarcinoma Treated with Primary Surgical Resection
Karapetyan L1, Hough B2, Geynisman DM3, Nason KS4, Luketich JD4,Jobe B5, Zaidi A5 and Gibson MK1*
1Department of Internal Medicine, Michigan State University, USA
2Cancer Institute, St. Joseph’s Hospital, USA
3Department of Medical Oncology, Fox Chase Cancer Center-Temple University Health System, USA
4Department of Cardiothoracic Surgery, University of Pittsburgh, USA
5Institute for the Treatment of Esophageal and Thoracic Disease, Allegheny Health Network, USA
*Corresponding author: Michael K. Gibson, Department of Internal Medicine, UH Case Medical Center, Case Western Reserve University, 11100 Euclid Avenue, Lakeside 1242, Cleveland, USA
Received: July 09, 2016; Accepted: August 08, 2016; Published: August 10, 2016
Abstract
Introduction: Patients with esophageal adenocarcinoma (EAC) preceded by Barrett’s esophagus (BE) may present at an earlier stage than those with EAC without BE. Previously, overall survival (OS) in patients +/- BE who underwent primary resection differed; due to different stages at presentation. Pretreatment stratification of patients based on prognosis may enable tailoring of therapy resulting in more favorable efficacy and toxicity. This study aimed to determine whether BEA and/or tumor location might serve as prognostic factors.
Methods: The retrospective review evaluated 363 patients with EAC treated with surgery at the Heart, Lung and Esophageal Surgery Institute (HLESI) of the U. of Pittsburgh between January 2000 and April 2008. IRB approval was obtained for data extraction from the HLESI database. Patients who had neoadjuvant therapy were excluded. Patients were assigned to the BEA group if the pathologist used the words, ‘Barrett’s esophagus’, or ‘intestinal metaplasia’.
Results: A total of 363 patients had esophagectomies. 227 patients were assigned to BE group and 136 to non- BE. Pathological stages were: Stage 0 (n=9), Stage I (n=101), Stage II (n=94), Stage III (n=139), and Stage IV (n=20). Median OS for whole cohort was 38 months, and higher pathological stages were associated with worse survival (HR 2.2 95% CI 1.8-2.6). Median OS for BE group was 45.3 months, and 21.2 months for non-BE (HR 0.57, 95% CI 0.42- 0.77). After controlling for stage in multivariate analysis, there was no survival difference in two groups (HR 0.99, 95% CI 0.72-1.4).
Conclusions: As expected OS worsened as stage increased. By univariate analysis, the median OS for BE was superior to non-BE. However, when controlled for stage, this difference disappeared. This larger series reinforces previous data showing an improved survival for BE vs non-BE that is mitigated by surgical stage.
Keywords: Esophageal adenocarcinoma; Barret’s esophagus; Overall survival
Introduction
Esophageal cancer comprises 1.5% of total cancer cases in the United States. The incidence of adenocarcinoma has been drastically increased with the most common tumor locations at the distal esophagus, gastroesophageal junction (GEJ) and gastric cardia [1]. The majority of patients present with locally advance or metastatic disease with the cure rate up to 30% when multimodality therapy is used. While patients with resectable disease do better, the median survival of those patients treated with either neoadjuvant chemoradiation or peri-operative chemotherapy is still only 2-4.5 years [2-4]. There is a significant improvement in the survival rate reported from the SEER database; however survival rates remain low [5]. In other malignancies, subsets of cancers have been found that are more sensitive to treatments, or have a unique weakness that can be targeted with selected therapies.
Intestinal-type columnar metaplasia of distal esophagus, also known as Barret’s esophagus (BE), is an important precursor to esophageal adenocarcinoma (EAC). The prevalence of BE is at 5.6 % in the United States, and the risk of transferring to EAC is less than 0.5% [6,7]. In an effort to identify subsets of patients with EAC that demonstrated differential behaviors, we undertook a single institution retrospective study to identify if the presence or absence of Barrett’s esophagus affects the survival of patients with EAC treated with primary surgical resection. We hypothesized that patients with evidence of intestinal metaplasia on their final pathology who underwent primary resection, had an improved survival when compared to those patients without evidence of Barrett’s esophagus. The operative volume at our center made us able to identify a larger cohort of patients, thus enabling a stronger evaluation of the impact of the presence of BEA (stratified by surgical stage) on survival. If BE adenocarcinoma (BEA) has an improved prognosis vs non BEA (NBEA), regardless of stage, those with NBEA might receive a different type of therapy than those with BEA. The data from this study are based on surgical pathology from patients treated with primary surgery, so if a difference exists, validation of this would need to be done on pre-surgical samples in order to enable treatment decisions based on this stratification factor.
Methods
After obtaining a HIPPA exempt waiver from the University of Pittsburgh Institutional Review Board, we reviewed medical records of 795 patients who underwent esophagectomyin Heart, Lung and Esophageal Surgery Institute (HLESI) at the University of Pittsburgh Medical Center (UPMC) hospitals between January 2000 and April 2008. These 795 patients included those who had esophagectomy for both adenocarcinoma, non-adenocarcinoma malignancies (e.g., squamous cell and lymphoma) and benign causes. 323 of those patients had either esophagectomies for benign causes or had a portion of their esophagus removed as part of a gastric operation. 109 of the remaining patients had neoadjuvant treatment, either with chemotherapy, radiation therapy, or both. There were 363 remaining patients who had adenocarcinoma of either the esophagus or GE junction who had not received pre- or post-op chemotherapy or radiation. Once identified, we performed an electronic chart review, making note of the patient’s age, date of surgery, pathologic stage, presence or absence of intestinal metaplasia on the final pathology specimen, and whether the tumor was esophageal or GE junction. Next we used the social security death index (SSDI) and performed a review of the electronic records to ascertain the vital status of each patient. Lastly, we submitted this dataset to the Cancer Registry to check our vital status results. Survival was determined by the method of Kaplan and Meier. Survival curves were compared using the logrank test. A p< 0.05 was considered statistically significant. Univariate and multivariate Cox proportional hazard models were used to investigate the role of clinical co-variates. Hazard ratios are expressed as mean with 95% confidence intervals.
All statistical analyses were done using Intercooled Stata version 10 (Stata Corporation, College Station, TX).
Results
Of the 363 evaluable patients, 227 had BEA and 136 had NBEA. Characteristics of all evaluated patients are listed in Table 1. The median overall survival (OS) for the whole cohort of 363 patients was 38.0 months (range: 31.4-45.0 months). Higher stages correlated with worse OS (HR 2.2 95% CI 1.8-2.6). Median OS for BEA was worse than in NBEA group (45.3 months vs 21.2 months, HR 0.57, 95% CI 0.42- 0.77) (Table 2). Their respective confidence intervals were non-overlapping and the log rank test showed a Pr >chi2 value of 0.0002. Figure 1, comparing survival in BEA vs NBEA group, shows statistically significant difference in these two groups (p=0.002). In contrast, when both groups were stratified by stage, there was no difference between BEA vs NBEA. HR for survival BEA/NBEA controlled for stage (HR 0.99, 95% CI 0.72-1.4). Median OS in GEJ was 24 months, and 32 months when tumor was located in esophagus (HR 1.15, 95% CI 0.85-1.6).
Characteristic
Median (range) or n %
Age, years
66.8 years (33.4-89.4)
Sex
309 (85.1%) male, 54 (14.9%) female
Tumor location
205 (56.4%) GEJ,
158 (44.6%) Distal/Other
AJCC Stage
Stage 0
9 (2.48 %)
Stage I
101 (27.8%)
Stage II
94 (25.9%)
Stage III
139 (38.3%)
Stage IV
20 (5.51%)
Table 1: Patient baseline characteristics.
Characteristic
BE group N (%)
Non-BE Group N (%)
Total
227(62.5%)
136(37.5%)
Median Age (y)
67.2
65.5
Stage 0
9 (4%)
0
Stage I
89(40%)
12(9%)
Stage II
63(28%)
31(23%)
Stage III
60(26%)
79(58%)
Stage IV
6(3%)
14(10%)
GE Junction
102(%)
103(%)
Non-GE Junction
125(%)
33(%)
Table 2: Comparison of Barret’s esophagus (BE) and non-BE groups.
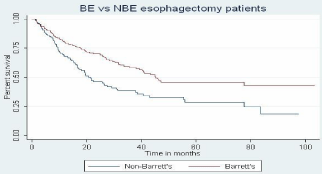
Figure 1: Kaplan-Meier survival analysis of overall survival plotted as
probability of surviving versus time from surgery in BE vs non-BE groups.
(P< 0.05).
Factor
HR
95% CI of HR
BE vs NBE non adjusted
0.57
1.8-2.6
Pathological Stage
0.99
0.72-1.4
Location (Esophagus vs GEJ)
1.15
0.85-1.6
Table 3: The results from multivariate Cox regression on OS.
Discussion
Adenocarcinoma of the esophagus (EAC) has a poor prognosis with the rising incidence in the Unites States. For patients with locally advanced disease, surgical treatment, usually with chemotherapy, radiation, or both offers the best hope for cure. Unfortunately, there is considerable toxicity associated with both the surgery and the neoadjuvant or adjuvant treatment, making both greater efficacy and reduced toxicity paramount.
In an effort to individualize care, newer research protocols have begun to separate adenocarcinoma from squamous cell carcinoma. Squamous cell carcinoma can be cured by primary chemoradiation therapy (CRT), in contrast, adenocarcinoma requires surgical resection as part of curative treatment [8]. Additionally, in the future we may further subdivide those patients who have undergone neoadjuvant treatment and have received a pCR versus those with persistent disease or progression into additional treatment or closer monitoring given what we know about their improved survival rates after surgery [9].
We conducted a retrospective study to evaluate the effect of presence/absence of BE on EAC survival. As expected OS worsened as stage increased. By univariate analysis, the median OS for BE was superior to non-BE. However, when controlled for stage, this difference disappeared. Portale, et al. evaluated this question previously, but it was done on a smaller sample size analyzing 215 patients. They found that while there appeared to be a survival advantage to the Barrett’s esophagus (BEA) group (5-year survival 61% vs 28%, P< .001), there was no significant difference while stratifying both groups by stage (Stage II 57.6% vs 61.7%, P = 0.89, and Stage III 19.6% vs 10.4%, P = 0.45, respectively). Patients in BEA group had more differentiated, less invasive, node negative tumor, and tend to present in less advanced clinical stages [10]. In a study of Lada, et al., a preoperative diagnosis of BE was a significant predictor of OS. However, again, when adjusting for stage, there was no statistically significant difference between presence/absence of BE (HR, 0.79; 95% CI, 0.53-1.61; P = 0.225) [11].
Our study reinforces previous data, suggesting that there is a relationship between BE and the stage at presentation or detection. One possible explanation for this phenomenon is a greater frequency of GERD symptoms in cases with BEA compared to NBEA. Another possible explanation is the potential disparity with regards to Barrett’s screening. Our study did not investigate the symptoms at presentation nor did it evaluate the number of patients in the Barrett’s group who were diagnosed by GERD screening. Further evaluation into this epidemiologic anomaly might include a more detailed investigation of the history of GERD symptoms in the respective groups and the percent of patients who were diagnosed by GERD screening.
Another possible confounder in our study is the suggestion that adenocarcinoma can occasionally “grow-over” the pre-existing Barrett’s esophagus making it appear as though the tumor was not associated with Barrett’s. This may reflect a more aggressive subset of Barrett’s associated adenocarcinoma. We could think of no easy means to separate one set from another. As gene expression profile data become more robust in esophageal cancer, perhaps those cancers that started out as Barrett’s may retain a certain signal that persists into the adenocarcinoma stage. Until then, there is the distinct possibility that a portion of our NBEA patients did in fact have preceding Barrett’s.
References
- Siegel R, Miller K D, Jemal A. Cancer Statistics, 2015. CA Cancer J Clin. 2015; 65: 5-29.
- Forastiere AA, Heitmiller RF, Lee DJ, Zahurak M, Abrams R, Kleinberg L, et al. Intensive chemoradiation followed by esophagectomy for squamous cell and adenocarcinoma of the esophagus. Cancer J Sci. Am. 1997; 3: 144-152.
- Heath EI, Burtness BA, Heitmiller RF, Salem R, Kleinberg L, Knisely JP, et al. Phase II evaluation of preoperative chemoradiation and postoperative adjuvant chemotherapy for squamous cell and adenocarcinoma of the esophagus. J Clin Oncol. 2000; 18: 868-876.
- Kleinberg L, Knisely JP, Heitmiller ES, Zahurak M, Salem R, Burtness B, et al. Mature survival results with preoperative cisplatin, protracted infusion 5-fluorouracil, and 44-Gy radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2003; 56: 328-334.
- Howlader N, Noone A M, Krapcho M. SEER Cancer Statistics Review, 1975-2012. National Cancer Institute. 2014.
- Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C, et al. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010; 23: 451-457.
- Wani S, Puli SR, Shaheen NJ, Westhoff B, Slehria S, Bansal A, et al. Esophageal adenocarcinoma in Barrett’s esophagus after endoscopic ablative therapy: a meta-analysis and systematic review. Am J Gastroenterol. 2009; 104: 502-513.
- Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999; 281: 1623-1627.
- Berger AC, Farma J, Scott WJ, Freedman G, Weiner L, Cheng JD, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005; 23: 4330-4337.
- Portale, G, Peters JH, Hagen JA, DeMeester SR, Gandamihardja TAK, Tharavej C, et al. Comparison of the Clinical and Histological Characterstics and Survival of Distal Esophageal-Gastroesophageal Junction Adenocarcinoma in Patients with and without Barrett Musca. Arch Surg. 2005; 140: 570-575.
- Lada MJ, Nieman DR, Han M, Timratana P, Alsalahi O, Peyre CG, et al. Gastroesophageal reflux disease, proton-pump inhibitor use and Barrett’s esophagus in esophageal adenocarcinoma: trends revisited. Surgery. 2013; 154: 856-866.