
Special Article - Multiple Myeloma
Ann Hematol Oncol. 2016; 3(7): 1102.
Proteasome Inhibitors in the Treatment of Multiple Myeloma
Raghupathy R1 and Margaret HL Ng2,3*
1Department of Clinical Oncology, Chinese University of Hong Kong, China
2Department of Anatomical and Cellular Pathology, Chinese University of Hong Kong, China
3State Key Laboratory in Oncology in South China, Chinese University of Hong Kong, China
*Corresponding author: Margaret HL Ng, Department of Anatomical and Cellular Pathology, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR, China
Received: July 05, 2016; Accepted: August 20, 2016; Published: August 23, 2016
Abstract
Proteasome inhibitors have become indispensable in myeloma management in transplant eligible and ineligible patients in both induction and salvage settings. Excellent efficacy and a manageable toxicity profile haves made these agents the backbone of myeloma care. After the first in class drug bortezomib was approved, several newer agents have been developed. The parenteral second generation drug carfilzomib has less peripheral neuropathy incidence than bortezomib. Oral proteasome inhibitors such as ixazomib, oproxomib and marizomib attempt to overcome the administration difficulties of the parenteral agents and have a differentiated toxicity profile. This review will focus on the role of proteasome inhibitors in myeloma treatment.
Keywords: Multiple myeloma; Proteasome; Bortezomib; Carfilzomib; Apoptosis; Ixazomib
Abbreviations
MM: Multiple Myeloma; ASCT: Autologous Stem Cell Transplant; PI: Proteasome Inhibitors; BMSC: Bone Marrow Stromal Cells; VLA4: Very late antigen 4; VCAM1: Vascular Cell Adhesion Molecule 1; CXC: Chemokine Receptor Type 4; CXCR4; CXCL12: Motif Chemokine 12; IL6: Interleukin 6; NF-kB: Nuclear Factor Kappa B; UPR: Unfolded Protein Response; SNP: Single Nucleotide Polymorphism; PFS: Progression Free Survival; OS: Overall Survival; RRMM: Relapsed Refractory Multiple Myeloma; ORR: Overall Response Rate; VMPT: Velcade Melphalan Prednisone, Thalidomide
Introduction
The landscape of MM treatment was revolutionized with the introduction of novel agents. Till the late 1990s treatment options for myeloma were limited to steroids, alkylators, anthracyclines and ASCT. The first breakthrough in MM management with novel agents came in 1999 when Singhal, et al. demonstrated the efficacy of thalidomide in the relapsed refractory disease [1]. Since then multiple new agents have been approved for myeloma management including proteasome inhibitors (PI), newer immunomodulators, monoclonal antibodies and epigenetic therapies. This review will address the role of PI in the management of MM and the potential mechanisms of drug resistance to this class of agents.
Basis of proteasome inhibition as a therapeutic strategy in MM
MM is a neoplasm arising from terminally differentiated, long-lived plasma cells which are responsible for immunological memory. Initial oncogenic mutations in myeloma appear to arise in the germinal center during B cell somatic hypermutation and antibody class switching; late oncogenic events including additional mutations and epigenetic changes occur after differentiation into plasma cells [2]. In addition to oncogenic mutations in the plasma cells, the bone marrow microenvironment, through direct cellular interactions and indirect effects mediated by cytokines, also plays an important role in myelomagenesis [3]. MM cells adhere to BMSC in the microenvironment. This adhesion is mediated by different molecules on the surface of MM cells and BMSC. VLA4 on MM cells binds to VCAM1 on BMSC and CXCR4 on MM cells bind to SDF- 1 (CXCL12) on BMSC [4,5]. These interactions between MM cells and BMSC stimulate IL-6 production by the BMSC [6]. IL-6 is a key growth factor for myeloma cells promoting their proliferation [7]. The production of IL-6 by BMSC is at least in part mediated by the transcription factor NF-kappa B [4].
The proteasome complex has an essential role in processing of the NF-kB1 precursor protein and activating NF-kB [8]. It also modulates expression and degradation of various adhesion molecules [9]. The intracellular ubiquitin proteasome pathway is responsible for degradation of 80-90% of intracellular dysfunctional proteins. In addition it modulates the turnover of key proteins involved in cell cycle progression and apoptosis [10-12]. While the proteasome complex is responsible for maintaining homeostasis in normal cells, cancer cells appear more susceptible to the inhibition of this complex than normal cells. Compared to peripheral blood mononuclear cells or bone marrow cells of normal controls, myeloma cell lines and patient samples showed about 170-fold greater sensitivity to effects of bortezomib mediating apoptosis [13]. This differential sensitivity of cancer cells has in part been attributed to NF-kB activation in cancer cells. Therefore targeting the proteasome is an attractive strategy for the treatment of myeloma.
First in class proteasome inhibitor: Bortezomib
Mechanisms of action of bortezomib: Bortezomib, a dipeptide boronic acid analogue, reversibly inhibits the 26S proteasome subunit, disrupting various signaling pathways and NFkB function resulting in cell cycle arrest and apoptosis [13,14]. Transcriptional and protein level changes of apoptotic regulators occur with bortezomib therapy. Gene expression profiling in bortezomib treated multiple myeloma cell lines have shown bortezomib mediated transcriptional upregulation of multiple proapoptotic molecules including pro caspase-8, pro-caspase-1, pro-caspase-7, caspase-4, caspase-9, and pro-caspase-5. Propoptotic BAX and BIM are stabilized. Transcription of antiapoptotic BCL2 and BIRC3 are decreased [15]. Bortezomib also functionally activates the intrinsic and extrinsic apoptotic pathways. These functional effects of bortezomib at protein level mostly occur through modulation of the ubiquitin proteasome pathway. Inhibition of proteasomal degradation of IkB by bortezomib results in cytoplasmic sequestration of prosurvival transcription factor NFkB and decreased cell proliferation [13]. Lack of proteasomic degradation of misfolded proteins results in their sequestration in the endoplasmic reticulum, activating a stress signaling pathway called the unfolded protein response (UPR) which inhibits cell cycle progression and activates apoptosis through the proapoptotic target CHOP/GADD 153 [16]. Bortezomib selectively inhibits proteasomedependent degradation of proapoptotic p53, p21, Noxa, and TRAIL receptors DR4 and DR5 [17]. Together these effects of bortezomib appear to cause apoptosis of the malignant plasma cells. Plasma cells appear uniquely sensitive to PI since their ability to turnover proteins is reduced during the differentiation process [18] (Figure 1).
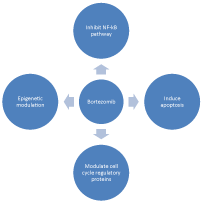
Figure 1: Important mechanisms of action of bortezomib.
Indication
Bortezomib combination
References
Upfront therapy for transplant eligible
Bortezomib, lenalidomide and low dose dexamethasone
[74,75]
Bortezomib, adriamycin and low dose dexamethasone
[36]
Bortezomib, thalidomide and low dose dexamethasone
[76]
Bortezomib, cyclophosphamide and low dose dexamethasone
[52]
Upfront therapy for transplant ineligible
Bortezomib, melphalan and prednisolone
[77]
Bortezomib, cyclophosphamide and dexamethasone
[52]
Bortezomib, thalidomide and prednisolone
[78]
Table 1: Bortezomib combination therapy in myeloma: important clinical trials summary.
Clinical development of bortezomib: Bortezomib (PS-341) was the first in class PI to be approved for the treatment of myeloma in the year 2003. In preclinical studies, bortezomib was found to inhibit cellular proliferation in 60 different cell lines derived from various human tumors [19]. Subsequent in vivo studies in myeloma xenograft models confirmed the efficacy of the drug in shrinking tumor tissue [20]. In phase I trials bortezomib proved safe and efficacious both as single agent and in combination with liposomal doxorubicin with a maximum tolerated dose of 1.3 mg/m2 administered intravenously on days 1,4,8 and 11 of a 21 day cycle [21,22]. Pharmacokinetic studies showed that after IV administration of bortezomib, about 83% is bound to plasma proteins and the drug is also widely distributed in the tissues. Metabolism occurs via the cytochrome P450 pathway in the liver and the drug is converted to inactive deboronated form. The plasma half-life of the drug after a single dose is 9 to 15 hours. The elimination mechanisms have not been clearly characterized. Two large phase II trials, SUMMIT and CREST were conducted in the relapsed refractory myeloma population. In the SUMMIT trial 202 patients with relapsed refractory MM showed a 35% ORR to single agent therapy with 4% achieving a PR and 6% achieving near CR [23]. The CREST trial included 67 patients with relapsed refractory MM and showed that reducing the bortezomib dose to 1 mg/m2 could still retain therapeutic benefit [24]. Combination phase II clinical trials using doxorubicin and dexamethasone or dexamethasone alone with bortezomib significantly increased the RR with ORR approaching 80-90% [25,26]. Initial FDA approval for the drug was based on the large Phase III randomized trial comparing boretzomib to high dose dexamethasone in relapsed refractory myeloma patients who had received 1-3 prior lines of therapy. In this APEX study 669 patients were randomized to bortezomib or dexamethasone with ability to crossover; bortezomib was proven superior to dexamethasone in terms of ORR, time to progression and OS [27]. Bortezomib has since been evaluated in multiple settings in different combinations in upfront and salvage therapy of both transplant eligible and ineligible patients and shown dramatic results making it the mainstay of therapy for the disease [28]. A summary of important trials and their findings has been presented in Table 1.
Bortezomib in high risk myeloma patients: 15-20% of myeloma patients have high risk disease at diagnosis based on tumor biology, disease burden, presence of renal failure and genetic characteristics [29]. Bortezomib is an effective agent in patients with renal failure given that the drug is not cleared via renal route and no dose adjustment is required. Rapid reversal of renal failure may occur in about 50% of myeloma patients presenting with renal failure by use of bortezomib [30]. High risk cytogenetic features in myeloma include t(4;14), t(14;16), t(14:20), del(17/ 17p), 1q 21 amplifications, 1 p deletions, karyotype del 13, and non hyperdiploid karyotype [31- 33]. Novel agents have been evaluated in these high risk cytogenetic subgroups and while outcomes remain inferior to standard risk patients, improvements have been noted in response rates and survival. In patients with t(4:14), bortezomib based induction, consolidation and maintenance along with autologous stem cell transplant almost completely obviates the adverse effect of the genetic mutation and improves PFS and OS compared to conventional therapies [34-36]. Deletion 17p remains challenging. In the HOVON65 trial where PAD induction (borteomib adriamycin dexamethasone) was compared to VAD (Vincristine, Adriamycin, dexamethasone) before single or tandem autologous stem cell transplant; the bortezomib based regime was shown to improve PFS and OS in 17p deletion, although not completely obviate the effect [37]. On the contrary the IFM 2005- 01 trial evaluating bortezomib dexamethasone and the GEM 2005 trial evaluating bortezomib thalidomide dexamethasone against conventional therapy before ASCT were unable to detect a benefit of bortezomib based therapy in 17p deletion [38,39]. Lenalidomide maintenance may offer marginal benefit to those with 17p deletion, and improved PFS in t(4:14) and del (17) patients [40]. The 2016 IMWG consensus statements suggests that combining a proteasome inhibitor with lenalidomide and dexamethasone greatly reduces the negative impact of t(4:14) and del (17) and should be considered for upfront management of patients with high risk myeloma followed by single or tandem high dose melphalan and ASCT and maintenance with PI and lenalidomide combination. Allogeneic transplant maybe considered in the setting of a clinical trial in younger patients [33,41,42]. Patients with multiple adverse cytogenetic abnormalities, especially those with 3 or more clonal abnormalities do not appear to benefit from these novel agents and have an estimated OS less than 2 years. Managing these patients remains an urgent unmet need in myeloma care.
Bortezomib effect on bone density: MM is characterized by imbalanced bone remodeling and increased osteoclastic activity resulting in lytic bone lesions [43]. Bortezomib has been shown to have an anabolic effect on bones in myeloma. In vitro studies co-culturing MM cells with preosteocytes and osteocytes showed evidence of autophagic cell death of the osteocytes due to the MM cells. Addition of bortezomib mitigated this effect and potentiated the effect of parathromone on bone formation [44].
Toxicities of bortezomib: Asthenia and mild gastrointestinal side effects of nausea, constipation and anorexia are reported in more than 40% of patients receiving bortezomib. The most disabling side effect requiring dose modification is peripheral neuropathy; grade 3 or 4 neuropathy can occur in up to 10% of patients receiving the drug and even higher incidence is noted when combined with other neurotoxins such as thalidomide [45]. Neuropathy is dose dependent, typically occurs by cycle 5 and incidence thereafter reaches a plateau. The mechanism of peripheral neuropathy caused by bortezomib remains unclear. Bortezomib accumulates in dorsal root ganglia of the nerves and also causes dysregulation of calcium homeostasis in mitochondria which have been postulated as pathogenic [46]. Gene expression profiling studies and SNP analyses from pretreatment myeloma samples in the HOVON 65 study suggest that myeloma characteristics and patient genetic composition may also influence the risk of neuropathy with bortezomib [47]. A SNP in the PKNOX1 gene, namely rs2839629 has recently been shown to have an association with bortezomib induced peripheral neuropathy [48]. The incidence of neuropathy has been reduced significantly by adopting once weekly dosing and subcutaneous administration of the drug [49,50]. About 30% of the patients develop transient thrombocytopenia, neutropenia is less common and occurs in 10-30% of treated patients [27]. Patients receiving bortezomib should receive prophylaxis against zoster reactivation [51].
Alternative bortezomib administration regimes: The significant neurotoxicity of the drug prompted evaluation of different dosing regimens to reduce the adverse events. In the randomized phase III GIMEMA study where VMPT followed by VT maintenance was compared to VMP, the VMPT arm was superior in terms of CR and PFS but incidence of grade 3 /4 neurotoxicity was significant at 28%, prompting about 15% of the patients to discontinue therapy. A protocol modification was then made to administer once weekly bortezomib to patients on both arms. Post-hoc analysis comparing once versus twice weekly dosing of bortezomib showed no significant difference in outcomes. However the incidence of grade 3 /4 neuropathy was significantly reduced to 8% in the once weekly arm compared to 28% in the twice weekly arm. (P< 0.001) [49]. In a phase 2 study evaluating the CyBorD regimen with once versus twice weekly bortezomib, a higher dose of 1.5 mg/m2 was used in the once weekly regimen compared to 1.3 mg/m2 in the twice weekly regimen. The total bortezomib dose per cycle was higher in the once weekly versus twice weekly regimen (6 mg/m2 vs. 5.2 mg/m2) however neuropathy rates were similar [52]. In a phase 3 non inferiority trial, of 222 patients randomized in a 2:1 fashion to subcutaneous versus intravenous bortezomib in a twice weekly regimen, no significant difference in efficacy was observed. Subcutaneous dosing was associated with a significantly lower risk of peripheral neuropathy of any grade (38% vs. 53%; p=0·044), and grade 3 or worse (6% vs. 16%; p=0·026). Most centers now adopt subcutaneous once weekly dosing of bortezomib in all regimens.
Resistance to bortezomib: Bortezomib is a very effective first line and salvage therapy for MM. However about 30% of patients are resistant to this drug. Different mechanisms have been proposed for Bortezomib resistance. Proteasome inhibitor resistant NFkB activity has been described [53]. Mutations in the bortezomib binding proteasome b5 subunit (PSMB5) have also been noted in resistant MM cell lines [54]. Studies have been performed in myeloma cell lines evaluating different anti and proapoptotic molecules and their effects on bortezomib resistance. The mucin 1 C-terminal subunit (MUC1-C) oncoprotein is aberrantly expressed in most MM cells, upregulates expression of p53-inducible regulator of glycolysis and apoptosis (TIGAR) and facilitates myeloma cell survival [55,56]. Inhibition of MUC1 has shown synergy with bortezomib treatment in myeloma cell lines. Myeloid cell leukemia-1 (Mcl-1) is another prosurvival molecule belonging to the bcl-2 family, targeting of which, in cell lines, results in c-Jun upregulation and MM cell death [57]. Tight junction protein 1(TJP1) suppresses the EGFR/JAK1/ STAT3 pathway which in turn decreases proteasome activity and increases sensitivity to proteasome inhibition [58]. Higher levels of TJP1 appear to correlate to increased proteasome sensitivity, although its role in drug resistance remains to be explored. Multiple myeloma cells are greatly dependent on the UPR pathway to relieve stress on the ER given the large amounts of paraproteins produced. XBP-1 is a transcriptional regulator of the UPR and low levels of this protein are associated with resistance to bortezomib [59]. Targeting bcl-2 with venetoclax has shown to improve response rates in patients with myeloma receiving bortezomib and dexamethasone [60].
Genome wide association studies Certain have shown certain micro RNA profiles to be correlated with bortezomib resistance. miR-29B targets PSME4 that encodes the proteasome activator PA 200. Synthetic miR-29B replacement inhibits PA200 aggregation with proteasomes, reduces the proteasome function and also acts synergistically with bortezomib in inhibiting proteasome function. Members of the miR-29B family also have epigenetic activity in hematological malignancies [61]. Comparison of bortezomib resistant versus naïve myeloma cells have shown decreased levels of miRNA29B in resistant cells and replacement of miRNA29B may be explored as a therapeutic option in this setting [62-64].
Second generation proteasome inhibitors
Carfilzomib: Carfilzomib is an epoxyketone, selective and irreversible proteasome inhibitor. It appears to have more selectivity for the chymotrypsin site of the proteasome and less off-target effects compared to bortezomib [65]. In 266 relapsed refractory myeloma patients treated with 1 to 5 prior lines of therapy including bortezomib based regimens, an ORR of 23.7% was seen with a median duration of response of 7.8 months with single agent carfilzomib at a dose of 20 mg/m2 intravenously twice weekly for 3 of 4 weeks in cycle 1, then 27 mg/m2 for = 12 cycles [66]. The phase III ASPIRE study in the relapsed refractory setting randomized 792 patients to either lenalidomide or dexamethasone alone or in combination with carfilzomib. The addition of carfilzomib resulted in a significant improvement of ORR (87.1% and 66.7, p< 0.001) and PFS (26.3 vs. 17.6 months, p: 0.0001). No significant increase in toxicities or treatment discontinuation was observed [67]. In the ENDEAVOR randomized phase III study carfilzomib with dexamethasone was compared against bortezomib with dexamethasone in RRMM and the carfilzomib combination showed an increase in PFS compared to bortezomib (18.7 vs.9.4 months, p< 0.0001) Subgroup analyses showed persistence of the PFS benefit of carfilzomib irrespective of bortezomib pretreatment [68]. Common toxicities observed with carfilzomib include fatigue and cytopenias, peripheral neuropathy is less frequent. Carfilzomib is FDA approved for treatment of relapsed myeloma as single agent; in combination with dexamethasone alone or with lenalidomide. Carfilzomib has shown good efficacy and a manageable safety profile in combination with dexamethasone and lenalidomide or cyclophosphamide in upfront treatment of myeloma both in transplant eligible and the elderly [69,70].
Oral proteasome inhibitors
The need for parenteral administration and degradation of peptide proteasome inhibitors by proteases prompted research into developing non-peptide orally active compounds. Several oral PIs are now in clinical trials. Ixazomib and delanzomib are boronic acid derivatives, oproxomib is an epoxyketone and marizomib is a salinosporamide [71]. Ixazomib is the most advanced oral PI in the pipeline. In a large phase III randomized placebo-controlled trial of 722 patients with RR MM, ixazomib orally administered once a week with lenalidomide and dexamethasone was compared against lenalidomide and dexamethasone alone. The ixazomib combination arm was associated with significant improvement in PFS, rates of serious adverse events being similar between both arms. Ixazomib was associated with thrombocytopenia, skin rash, peripheral neuropathy and gastrointestinal side effects [72]. Ixazomib is now approved for treatment of RRMM after failing at least 1 prior therapy in combination with lenalidomide and dexamethasone. Marizomib may be administered either intravenously or orally and has specificity distinct from bortezomib and carfilzomib in 20S proteasome inhibition. In a phase 1b trial in RR MM patients, intravenous administration of marizomib was associated with clinical benefit and had a tolerable side effect profile [73]. Gastrointestinal toxicities and asthenia were observed. Combination studies of marizomib with pomalidomide and dexamethasone are now being conducted. Early phase trials of oproxomib in RRMM are also underway.
Conclusion
Proteasome inhibitors have greatly improved outcomes in myeloma patients. Patients with high risk myeloma are best treated with triple induction therapy comprising lenalidomide, bortezomib and steroid (RVD) followed by ASCT if eligible, RVD consolidation therapy and maintenance with both lenalidomide and bortezomib combination. Standard risk patients undergo similar RVD induction and ASCT if eligible, single agent lenalidomide maintenance appears adequate. Induction and maintenance regimes are modified based on host factors and tolerability. (Bianca C, JCO) Ultra-high risk myeloma patients with 3 or more cytogenetic abnormalities do not respond well to novel agents and optimal therapy for this group remains to be explored. Recently new monoclonal antibodies have been developed such as daratumumab against CD38 which shows efficacy in PI and immunomodulator refractory patients and elotuzumab against SLAMF-7 which has activity in combination with immunomodulators [2]. Immune checkpoint inhibitors are also being studied in myeloma with the promise of new breakthroughs for this hitherto incurable disease.
References
- Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999; 341: 1565-1571.
- Bianchi G, Richardson PG, Anderson KC. Promising therapies in multiple myeloma. Blood. 2015; 126: 300-310.
- Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007; 7: 585-598.
- Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996; 87: 1104-1112.
- Alsayed Y, Ngo H, Runnels J, Leleu X, Singha UK, Pitsillides CM, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007; 109: 2708-2717.
- Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol. 2011; 6: 249-274.
- Treon SP, Anderson KC. Interleukin-6 in multiple myeloma and related plasma cell dyscrasias. Curr Opin Hematol. 1998; 5: 42-48.
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994; 78: 773-785.
- Read MA, Neish AS, Luscinskas FW, Palombella VJ, Maniatis T, Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 1995; 2: 493-506.
- Rubin DM, Finley D. Proteolysis. The proteasome: a protein-degrading organelle? Curr Biol. 1995; 5:854-858.
- King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996; 274: 1652-1659.
- Shen M, Schmitt S, Buac D, Dou QP. Targeting the ubiquitin-proteasome system for cancer therapy. Expert Opin Ther Targets. 2013; 17: 1091-1108.
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001; 61: 3071-3076.
- Schwartz R, Davidson T. Pharmacology, pharmacokinetics, and practical applications of bortezomib. Oncology (Williston Park). 2004; 18: 14-21.
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002; 99: 14374-14379.
- Obeng EA, Carlson LM, Gutman DM, Harrington WJ,Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006; 107: 4907-4916.
- Liu FT, Agrawal SG, Gribben JG, Ye H, Du MQ, Newland AC, et al. Bortezomib blocks Bax degradation in malignant B cells during treatment with TRAIL. Blood. 2008; 111: 2797-2805.
- Cenci S, Mezghrani A, Cascio P, Bianchi G, Cerruti F, Fra A, et al. Progressively impaired proteasomal capacity during terminal plasma cell differentiation. EMBO J. 2006; 25:1104-1113.
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res.1999; 59: 2615-2622.
- LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002; 62: 4996-5000.
- Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002; 20: 4420-4427.
- Orlowski RZ, Voorhees PM, Garcia RA, Hall MD, Kudrik FJ, Allred T, et al. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005; 105: 3058-3065.
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003; 348: 2609-2617.
- Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004; 127: 165-172.
- Oakervee HE, Popat R, Curry N, Smith P, Morris C, Drake M, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol. 2005; 129: 755-762.
- Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005; 129: 776-783.
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005; 352: 2487-2498.
- Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011; 11: 239-253.
- Avet-Loiseau H. Ultra high-risk myeloma. Hematology Am Soc Hematol Educ Program. 2010; 2010: 489-493.
- Dimopoulos MA, Roussou M, Gavriatopoulou M, Zagouri F, Migkou M, Matsouka C, et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib-based regimens: identification of predictive factors. Clin Lymphoma Myeloma. 2009; 9: 302-306.
- Bianchi G, Richardson PG, Anderson KC. Best treatment strategies in high-risk multiple myeloma: navigating a gray area. J Clin Oncol. 2014; 32: 2125-2132.
- Lonial S, Boise LH, Kaufman J. How I treat high-risk myeloma. Blood. 2015; 126:1536-1543.
- Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016; 127: 2955-2962.
- Avet-Loiseau H, Leleu X, Roussel M, Moreau P, Guerin-Charbonnel C, Caillot D, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol. 2010; 28: 4630-4634.
- Cavo M, Pantani L, Petrucci MT, Patriarca F, Zamagni E, Donnarumma D, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012; 120: 9-19.
- Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012; 30: 2946-2955.
- Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012; 119: 940-948.
- Rosinol L, Oriol A, Teruel AI, Hernandez D, Lopez-Jimenez J, de la Rubia J, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012; 120: 1589-1596.
- Harousseau JL, Avet-Loiseau H, Attal M, Charbonnel C, Garban F, Hulin C, et al. Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 Trials. J Clin Oncol. 2009; 27: 5720-5726.
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012; 366: 1782-1791.
- Roussel M, Lauwers-Cances V, Robillard N, Hulin C, Leleu X, Benboubker L, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. J Clin Oncol. 2014; 32: 2712-2717.
- Lonial S, Nooka AK. Myeloma Is Not a Single Disease. J Oncol Pract. 2016; 12: 287-292.
- Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009; 23: 435-441.
- Toscani D, Palumbo C, Dalla Palma B, Ferretti M, Bolzoni M, Marchica V, et al. The Proteasome Inhibitor Bortezomib Maintains Osteocyte Viability in Multiple Myeloma Patients by Reducing Both Apoptosis and Autophagy: A New Function for Proteasome Inhibitors. J Bone Miner Res. 2016; 31: 815-827.
- Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010; 28: 4621-4629.
- Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008; 112: 1593-1599.
- Broyl A, Corthals SL, Jongen JL, van der Holt B, Kuiper R, de Knegt Y, et al. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: a prospective analysis of data from the HOVON-65/GMMG-HD4 trial. Lancet Oncol. 2010; 11: 1057-1065.
- Magrangeas F, Kuiper R, Avet-Loiseau H, Gouraud W, Guerin-Charbonnel C, Ferrer L, et al. A Genome-Wide Association Study Identifies a Novel Locus for Bortezomib-Induced Peripheral Neuropathy in European Patients with Multiple myeloma. Clin Cancer Res. 2016.
- Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010; 116: 4745-4753.
- Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011; 12: 431-440.
- Pour L, Adam Z, Buresova L, Krejci M, Krivanova A, Sandecka V, et al. Varicella-zoster virus prophylaxis with low-dose acyclovir in patients with multiple myeloma treated with bortezomib. Clin Lymphoma Myeloma. 2009; 9: 151-153.
- Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Laumann K, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. 2010; 115: 3416-3417.
- Markovina S, Callander NS, O'Connor SL, Kim J, Werndli JE, Raschko M, et al. Bortezomib-resistant nuclear factor-kappaB activity in multiple myeloma cells. Mol Cancer Res. 2008; 6: 1356-1364.
- Ri M, Iida S, Nakashima T, Miyazaki H, Mori F, Ito A, et al. Bortezomib-resistant myeloma cell lines: a role for mutated PSMB5 in preventing the accumulation of unfolded proteins and fatal ER stress. Leukemia. 2010; 24:1506-1512.
- Kawano T, Ahmad R, Nogi H, Agata N, Anderson K, Kufe D. MUC1 oncoprotein promotes growth and survival of human multiple myeloma cells. Int J Oncol. 2008; 33: 153-159.
- Yin L, Kosugi M, Kufe D. Inhibition of the MUC1-C oncoprotein induces multiple myeloma cell death by down-regulating TIGAR expression and depleting NADPH. Blood. 2012; 119: 810-816.
- Fan F, Tonon G, Bashari MH, Vallet S, Antonini E, Goldschmidt H, et al. Targeting Mcl-1 for multiple myeloma (MM) therapy: drug-induced generation of Mcl-1 fragment Mcl-1(128-350) triggers MM cell death via c-Jun upregulation. Cancer Lett. 2014; 343: 286-294.
- Zhang XD, Baladandayuthapani V, Lin H, Mulligan G, Li B, Esseltine DL, et al. Tight Junction Protein 1 Modulates Proteasome Capacity and Proteasome Inhibitor Sensitivity in Multiple Myeloma via EGFR/JAK1/STAT3 Signaling. Cancer Cell. 2016; 29: 639-652.
- Nikesitch N, Ling SC. Molecular mechanisms in multiple myeloma drug resistance. J Clin Pathol. 2016; 69: 97-101.
- Moreau P, Chanan-Khan A, Roberts AW, Agarwal AB, Facon T, Kumar S, et al. Safety and Efficacy of Venetoclax (ABT-199/GDC-0199) in Combination with Bortezomib and Dexamethasone in Relapsed/Refractory Multiple Myeloma: Phase 1b Results. Blood. 2015; 126: 3038.
- Amodio N, Rossi M, Raimondi L, Pitari MR, Botta C, Tagliaferri P, et al. miR-29s: a family of epi-miRNAs with therapeutic implications in hematologic malignancies. Oncotarget. 2015; 6:12837-12861.
- Amodio N, Bellizzi D, Leotta M, Raimondi L, Biamonte L, D'Aquila P, et al. miR-29b induces SOCS-1 expression by promoter demethylation and negatively regulates migration of multiple myeloma and endothelial cells. Cell Cycle. 2013; 12: 3650-3662.
- Amodio N, Di Martino MT, Foresta U, Leone E, Lionetti M, Leotta M, et al. miR-29b sensitizes multiple myeloma cells to bortezomib-induced apoptosis through the activation of a feedback loop with the transcription factor Sp1. Cell Death Dis. 2012; 3: e436.
- Jagannathan S, Vad N, Vallabhapurapu S, Vallabhapurapu S, Anderson KC, Driscoll JJ. MiR-29b replacement inhibits proteasomes and disrupts aggresome+autophagosome formation to enhance the antimyeloma benefit of bortezomib. Leukemia. 2015; 29: 727-738.
- Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ, et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011; 17: 2734-2743.
- Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012; 120: 2817-2825.
- Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015; 372: 142-152.
- Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hajek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016; 17: 27-38.
- Korde N, Roschewski M, Zingone A, Kwok M, Manasanch EE, Bhutani M, et al. Treatment With Carfilzomib-Lenalidomide-Dexamethasone With Lenalidomide Extension in Patients With Smoldering or Newly Diagnosed Multiple Myeloma. JAMA Oncol. 2015; 1: 746-754.
- Bringhen S, Petrucci MT, Larocca A, Conticello C, Rossi D, Magarotto V, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014; 124: 63-69.
- Allegra A, Alonci A, Gerace D, Russo S, Innao V, Calabro L, et al. New orally active proteasome inhibitors in multiple myeloma. Leuk Res. 2014; 38: 1-9.
- Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016; 374: 1621-1634.
- Richardson PG, Zimmerman TM, Hofmeister CC, Talpaz M, Chanan-Khan AA, Kaufman JL, et al. Phase 1 study of marizomib in relapsed or relapsed and refractory multiple myeloma: NPI-0052-101 Part 1. Blood. 2016; 127: 2693-2700.
- Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012; 119: 4375-4382.
- Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010; 116: 679-686.
- Moreau P, Avet-Loiseau H, Facon T, Attal M, Tiab M, Hulin C, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011; 118: 5752-5758.
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol. 2013; 31: 448-455.
- Mateos MV, Oriol A, Martinez-Lopez J, Gutierrez N, Teruel AI, de Paz R, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010; 11: 934-941.