
Case Report
Ann Hematol Oncol. 2016; 3(7): 1104.
Concurrent Esophageal Squamous Cell Carcinoma and Recurrent Hodgkin Lymphoma in an HIV Positive Patient: A Case Report
Bonder B¹, Karapetyan L², Turakhia S³, Sandhaus LM³ and Gibson MK4*
¹Department of Internal Medicine, Case Western Reserve University, USA
²Department of Internal Medicine, Michigan State University, USA
³Department of Pathology, Case Western Reserve University, USA
4Department of Hematology/Oncology, Case Western Reserve University, USA
*Corresponding author: Michael K. Gibson, Department of Hematology/Oncology, UH Case Medical Center, Case Western Reserve University, 11100 Euclid Avenue, Lakeside 1242, Cleveland, OH, 44106, USA
Received: July 06, 2016; Accepted: August 27, 2016; Published: August 29, 2016
Abstract
Patients with human immunodeficiency virus (HIV) infection have an increased risk of developing certain malignancies compared with the general population. HIV-specific risk factors such as viral co-infections, immunosuppression, and chronic inflammation contribute to development of cancer in HIV patients.
We present the case of an HIV-infected patient with concurrent recurrent stage IVB Hodgkin Lymphoma and newly diagnosed stage III esophageal squamous cell carcinoma. Metastases from both cancers were found in a single supraclavicular lymph node.
Keywords: Esophageal cancer; Hodgkin’s lymphoma; HIV; Metastases
Introduction
Individuals infected with HIV have a higher prevalence of certain malignancies compared with the general population. HIV-related immune suppression and decreased immune surveillance result in an increased risk of these cancers. The high prevalence of smoking and alcohol use along with viral infections in HIV patients further contribute to development of cancer. Initiation of highly active antiretroviral therapy (HAART) resulted in durable control of viral replication and increased life expectancy of HIV patients. As a result, the rates of AIDS defining malignancies, such as Kaposi sarcoma, cervical cancer and non-Hodgkin lymphomas, declined. However, the rates of non-AIDS defined cancers remained relatively constant despite improvement in ART. Anal cancer, Hodgkin’s lymphoma (HL), lung and liver cancers are the most common non- AIDS defined cancers in HIV patients [1,2]. The incidence of HL is eleven times higher in HIV-infected patients than in general population and in most cases is associated with the presence of oncogenic Epstein- Barr virus (EBV). Mixed cellularity (MC) is the most common histological subtype. HIV-HL patients often present in advanced stages of disease (stage III or IV) with extranodal involvement. High international prognostic score (IPS), stage III-IV disease, and CD4 cell count less than 200 cell/μl are associated with poor prognosis in HL-HIV patients [3,4].
Esophageal cancer (EC) comprises a small percentage (1.5%) of total cancer cases in the United States. Compared with the general population, the risk of EC is increased ([standardized incidence ratios] SIR, 1.69; 95% CI, 1.37-2.07) in people with acquired immunodeficiency syndrome (AIDS). The incidence is equally elevated for both adenocarcinoma and squamous cell cancer (SCC) of esophagus. The high prevalence of smoking and alcohol use in HIV patients contribute to the increased risk of EC in this population as well [5].
We present a case of HIV-infected patient who presented with recurrent stage IVB HL and advanced/stage III EC with both cancers present as metastases in a single supraclavicular lymph node.
Case Presentation
A 55-year-old Hispanic male presented with dysphagia, chronic sore throat, hoarseness and 20 pound weight loss over two months. He had HIV infection and was on HAART therapy. The CD4 count on presentation was 111 cells/μl, and the viral load was 20 copies/ ml. He had a history of stage IVB EBV positive HL for which he underwent six cycles of doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) two years prior to presentation-resulting in a complete response by imaging and bone morrow biopsy. Other comorbidities included: type II diabetes mellitus, gastro esophageal reflux disease (GERD), hypothyroidism, dyslipidemia, hypertension, diverticulosis and a trial fibrillation.
Initial work-up led to a vocal cord biopsy which was negative for cancer. Despite treatment with supportive care and antibiotics, his symptoms acutely worsened, and he underwent a CT of the neck and chest. This revealed a right supraclavicular lymph node/mass that measured 3.2×3.5 cm as well as asymmetrical thickening of the proximal esophagus. Subsequent esophagogastroduodenoscopy (EGD) showed esophageal stricture and a mass that started below the cricopharyngeus and extended inferiorly for approximately 4 cm in length. The stricture was dilated and a percutaneous endoscopic gastrostomy tube was placed. Biopsy of the mass was positive for invasive squamous cell carcinoma of esophageal origin. He was transferred to our facility for further cancer care.
Further staging by positron emission tomography (PET) scan showed hyper-metabolic thickening of the proximal esophagus with adjacent hyper-metabolic para-esophageal and supraclavicular adenopathy as well as extensive bulky retroperitoneal adenopathy (Figure 1). Biopsies of both nodal regions were performed. The periarotic lymph node showed classic EBV positive Hodgkin’s Lymphoma (HL). The supraclavicular lymph node contained both HL and metastatic squamous cell carcinoma (Figure 2). A bone marrow biopsy was done and also showed HL.
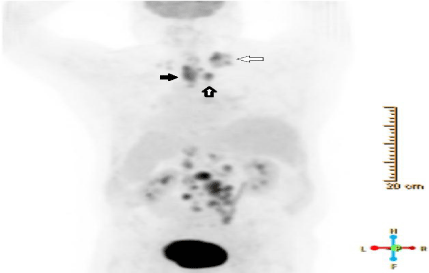
Figure 1: Staging Flourine-18 fluorodeoxyglucose PET scan demonstrating
hypermetabolic thickening of the proximal esophagus (black arrow, SUV
max 10.6), hypermetabolic paraesophageal (thick arrow, SUV max 10.1)
and supraclavicular (thin arrow, SUV max 10.7) adenopathy with extensive
retroperitoneal adenopathy (not labeled).
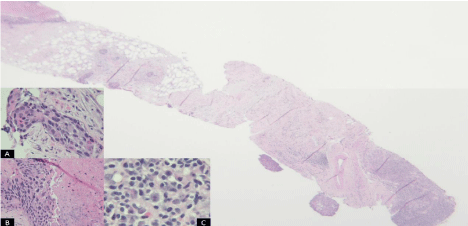
Figure 2: Low power (2X) showing lymphohistiocytic infiltrate (lower right) and
metastatic carcinoma with necrosis (upper left), Insert A: 40x of SCC, Insert B:
20x of SCC with associated necrosis, Insert C: 40x of with lymphohistiocytic
infiltrate with diagnostic Reed-Sternberg cell with prominent eosinophillic
nucleoli consistent with involvement by classical Hodgkin lymphoma.
The HL was staged as stage IV, and the esophageal cancer was staged as T3N1M0 due to tumor invasion to adventitia and spread to supraclavicular LN. Following presentation at multidisciplinary tumor board, treatment started first for esophageal cancer, using the CROSS approach of definitive chemoradiation therapy with carboplatin and paclitaxel. Given the known activity of these drugs against HL as well, we elected to initiate standard chemotherapy for HL after completion of treatment for esophageal cancer. The patient received 5 cycles of carboplatin and paclitaxel with total radiation dose of 41.4 Gy which was given in 23 fractions of 1.8 Gy each, with 5 fractions administered per week.
Unfortunately 2 months after diagnosis the patient presented with abdominal pain and was found to be bacteremic with imaging demonstrating extensive small bowel pneumatosis, portal and mesenteric vein gas. He underwent exploratory laparatomy out of concern for ischemic bowel, however no area of frank necrosis was observed. Following surgery he decompensated with multiple organ system failure and the family elected to withdraw care.
Discussion
The incidence of non-AIDS defining cancers is two to three times higher in HIV-infected patients than in the general population. Current guidelines recommend initiation of HAART therapy independent of the CD4 lymphocyte count. The optimal control of HIV replication is associated with increased overall survival and less comorbidities in these patients [6,7].
Despite optimal control, the risk of developing cancer remains high in patients who are on HAART and have a CD4 cell count >500/ μl and an undetectable viral load. In a study by Silverberg, et al. HIVinfected patients with a CD4 cell count >500/μl had 13.5 time higher risk of developing HL than non-HIV infected people [8]. Although HIV-HL patients often present with altered performance status and high frequency of B symptoms, standard chemotherapy with ABVD provides good results with complete response rates of 83% and overall survival rates of 78%. These results are similar to non-HIV patients. Olszewski, et al. showed that patients with HIV-positive status had a similar mortality to non-infected patients for classical histologic subtypes, including nodular sclerosing. IPS score >3 and CD4 count less than 200/μl were associated with decreased OS (HR, 1.84; P=.06 and HR, 2.04; P=.04, respectively) [9,10].
The risk of EC is not elevated in HIV patients without AIDS compared with the general population (SIR, 0.95; 95% CI, 0.53-1.56) [6]. Regardless of histology, approximately 50-60% of patients present with incurable locally advanced or metastatic disease. For those with locally advanced, non-metastatic disease, cure is achieved in up to 30% of patients when multimodality therapy is used [11].
This case demonstrates a number of observations that are relevant to clinical practice. Most importantly, a single core biopsy of the supraclavicular lymph node detected both malignancies. This unusual occurrence emphasizes the importance of obtaining adequate tissue for pathologic diagnosis and accurate staging. US-guided core biopsy of the supraclavicular lymph node was important for detecting metastatic EC. As a result, radiation therapy included node treatment as well. Treatment of concurrent cancers often represents a challenge for oncologist. Although HL is a rapidly growing cancer, EC presents as a highly lethal malignancy with poor prognosis with often being resistant to chemotherapy. Therefore, when faced with treating concurrent cancers, it is reasonable to treat the most lethal cancer first with a regimen that ideally has activity against both malignancies.
References
- Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst. 2015; 107.
- Rubinstein PG, Aboulafia DM, Zloza A. Malignancies in HIV/AIDS: From Epidemiology to Therapeutic Challenges. AIDS. 2014; 28: 453-465.
- Tirelli U, Errante D, Dolcetti R, Gloghini A, Serraino D, Vaccher E, et al. Hodgkin’s disease and human immunodeficiency virus infection: clinicopathologic and virologic features of 114 patients from the Italian Cooperative Group on AIDS and Tumors. J Clin Oncol. 1995; 13:1758-1767.
- Chimienti E, Spina M, Gastaldi R, Rossi G, Gabarre J, Talamini R, et al. Clinical characteristics and outcome of 280 patients (PTS) with Hodgkin’s disease and HIV infection (HD-HIV) in pre-and HAART (highly active antiretroviral era). Annals of Oncology. 2008; 19:136-137.
- Zhang YX, Gui XE, Zhong YH, Rong YP, Yan YJ. Cancer in cohort of HIV-infected population: prevalence and clinical characteristics. J Cancer Res Clin Oncol. 2011; 137: 609-614.
- Brugnaro P, Morelli E, Cattelan F, Petrucci A, Panese S, Eseme F, et al. Non-acquired immunodeficiency syndrome definings malignancies among human immunodeficiency virus-positive subjects: Epidemiology and outcome after two decades of HAART era. World J Virology. 2015; 4: 209-218.
- Cobucci RN, Lima PH, de Souza PC, Costa VV, Cornetta Mda C, Fernandes JV, et al. Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: a systematic review. J Infect Public Health. 2015; 8: 1-10.
- Silverberg MJ, Chao C, Leyden WA, Xu L, Horberg MA, Klein D, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011; 20: 2551-2559.
- Castillo JJ, Bower M, Brühlmann J, Novak U, Furrer H, Tanaka PY, et al. Prognostic factors for advanced-stage human immunodeficiency virus-associated classical Hodgkin lymphoma treated with doxorubicin, bleomycin, vinblastine, and dacarbazine plus combined antiretroviral therapy: A multi-institutional retrospective study. Cancer. 2015; 121: 423-431.
- Olszewski AJ, Castillo JJ. Outcomes of HIV-associated Hodgkin lymphoma in the era of antiretroviral therapy. AIDS. 2016; 30: 787-796.
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013; 381: 400-412.