
Special Article - Multiple Myeloma
Ann Hematol Oncol. 2016; 3(10): 1116.
Extramedullary Relapse at Diverse Sites in an Acute Myeloid Leukemia with Minimal Differentiation with Simultaneous Presence of t(4;11)(Q21;Q23) and t(7;11) (Q36;P11)
Shi QL¹, Yuan YH¹, Chen LJ¹*, Wu HX¹, Shen WY¹, Qian SX¹, Li JY¹ and Zhu YQ²*
¹Department of Hematology, First Affiliated Hospital of Nanjing Medical University, China
²College of Life Science, Nanjing Normal University, China
*Corresponding author: Chen Lijuan, Department of Hematology, First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital, No. 300 Guangzhou Road, Nanjing 210029, China Zhu Yongqiang, College of Life Science, Nanjing Normal University, No. 1 Wenyuan Road, Nanjing 210037, China
Received: July 21, 2016; Accepted: October 14, 2016; Published: October 17, 2016
Abstract
Nonrandom karyotypic changes have been revealed to play a key role in leukemogenesis. Specific karyotypic changes in leukemias have significant diagnostic and prognostic values, and are also associated with clinical manifestations. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a valid treatment option for high risk acute leukemias. Extramedullary relapse after allo-HSCT has been thought to be associated with a very poor prognosis. Here we present the case and associated literature review of an AML-M0 patient with simultaneous presence of t(4;11)(q21;q23) and a novel translocation t(7;11) (q36;p11) developed extramedullary relapse post-HSCT. t(4;11)(q21;q23) and t(7;11)(q36;p11) are adverse risk of AML-M0. FLAG is a good treatment regimen for this kind of leukemia.
Keywords: Acute myeloid leukemia; Leukemia cells; Extramedullary relapse
Introduction
Nonrandom karyotypic changes have been revealed to play a key role in leukemogenesis. Specific karyotypic changes in leukemias have significant diagnostic and prognostic values, and are also associated with clinical manifestations. Several translocations in myelogenous leukemia, such as t(9;22), t(15;17), t(8;21), etc., have been identified [1-3], leading to the detection of leukemia- specific gene abnormalities. However, many other karyotypic changes remain unclear.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a valid treatment option for a significant subset of patients with acute leukemias [4-6]. The curative effect of allo-HSCT is attributable to its ability to decrease leukemia relapse significantly compared to conventional or high-dose chemotherapy with or without autologous HSCT because of its graft-versus-leukemia (GVL) effect [7,8]. However, disease relapse after allo-HSCT still remains the major cause of treatment failure [9,10]. Extramedullary relapse after allo-HSCT has been thought to be associated with a very poor prognosis. Chromosomal aberrations, such as t(8;21), inv(16), MLL rearrangement are among the predisposing factors of isolated extramedullary relapse [11].
Here we report an AML-M0 patient with simultaneous presence of t(4;11)(q21;q23) and a novel translocation t(7;11) (q36;p11) developed extramedullary relapse post-HSCT and was sensitive to FLAG regimen (fludarabine 25 mg/m2/day and cytarabine 2 g/m2/ day by intravenous infusion on days 1 to 5 and G-CSF 300 μg/day by subcutaneous injection on days 0 to 5).
Case Presentation
A 44-year-old male had suffered from dizziness, fatigue, and exhibited purpura in the legs for about 2 weeks before being admitted to the first affiliated hospital of Nanjing Medical University. Peripheral blood examination showed hemoglobin concentration of 73 g/L, platelet count of 89×109/L, and white blood cell count (WBC) of 4.2×109/L with 5.2% myeloblasts, 20.4% myelocytes, 16.7% neutrophils, 57.7% lymphocytes, and no eosinophilic or basophilic cells. No hepatosplenomegaly or lymphoadenopathy was present. Bone marrow aspiration revealed a hypercellular marrow with 48.8% myeloblasts. Leukemia cells were negative for peroxidase, and weakly positive for periodic acid Schiff (PAS) stain. Flow cytometric analysis was positive for HLA-DR, CD117, CD33, CD13, CD61, CD11b. A diagnosis of acute myeloblastic leukemia with minimal differentiation (AML- M0) was made. The bone marrow cytogenetic analysis by routine R-banding technique after 24 hours culture without stimulation showed clonal abnormalities with the simultaneous presence of t(4;11)(q21;q23), t(7;11) (q36;p11) (Figure 1). This concurrent presence of 2 specific translocations was confirmed by multiplex fluorescence in situ hybridization (M- FISH).
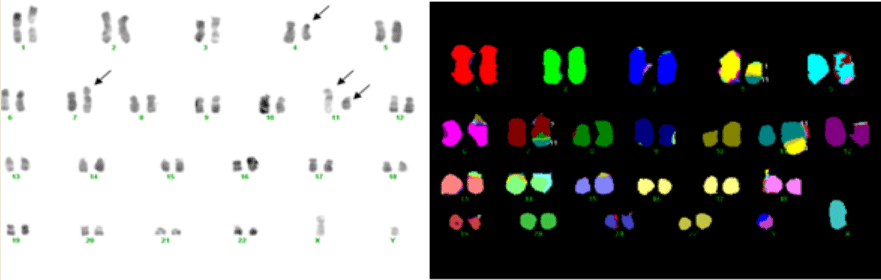
Figure 1: Multiplex fluorescence in situ hybridization.
An induction chemotherapy regimen of IA (idarubicin 12 mg/ m2/day by intravenous infusion on days 1 to 3 and cytarabine 100 mg/m2/day by continuous intravenous infusion on days 1 to 7) was administered, but it had no response. Thus, reduction with FLAG protocol was administered and complete remission was achieved, then he underwent allogeneic hematopoietic stem cell transplantation from a matched sibling sister, with Bu/Cy (busulfan 4 mg/kg/day orally on days -7 to -4 and cyclophosphamide 60 mg/ kg/day by intravenous infusion on days -3 to -2) as conditioning and cyclosporine A (CsA) 1.5 mg/kg/day from day-1 and methotrexate (MTX) (15 mg/m2 on day 1,10 mg/m2 on days 3, 6 and11) as graftversus- host disease (GVHD) prophylaxis. He engrafted with grade I acute GVHD.
Twenty-one months post-HSCT he presented left testicular mass. Orchiectomy of left testis was performed and the histopathology revealed myeloid sarcoma. 69.7% of testicular cells were positive for CD34, HLD-DR, CD117, CD13, CD33 and CD11b detected by flow cytometry. These staining findings confirmed the myeloid identity of the blasts. Bone marrow aspiration showed complete morphological remission and 100% donor chimerism by X/Y fluorescence in situ hybridization (FISH). He was treated with irradiation of testis, whole brain and spinal (24Gy), conventional chemotherapy (aclacinomycin 10 mg/d, d1-8; cytarabine 17 mg/d, d1-14, G-CSF 300 μg, qd) and DLI (total cell dose 1.7×108 /kg). At 39 months post- HSCT, biopsy-proven granulocytic sarcoma recurred in the skin of legs, arms and abdomen, subcutaneous tissues of neck, eyelids and penis (Figure 2). Cells were negative for peroxidase stain and the immunophenotype was MPO++, CD34++,CD43++, BCL- 2+, CD56+, CD3-,CD20-, CD45RO-, Pax-5-,TdT-,CD5-,TIA- for immunohistochemistry. X/Y FISH of granulocytic sarcoma cells showed that the remaining recipient cells were 68%, suggesting extramedullary relapse from recipient origin. A morphology examination of a bone marrow aspirate still showed no evidence of myeloid leukemia and a full donor chimerism was documented by FISH. A reduced- intensive FLAG regimen (fludarabine 25 mg/m2/ day and Ara-C 1g/m2/day by intravenous infusion on days 1 to 5 and G-CSF300 μg/day by subcutaneous injection on days 0 to 5) was administered and granulocyte sarcoma decreased significantly
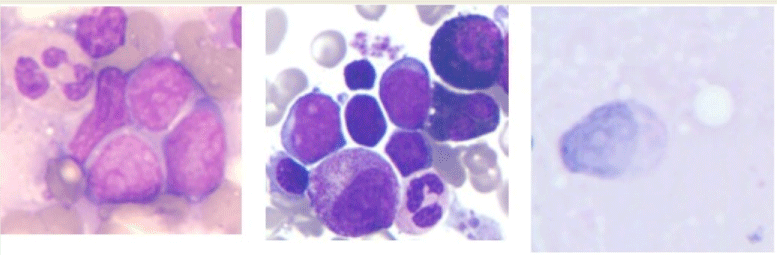
Figure 2: Skin biopsy showing infiltration of blast cells (×1000) A: Wright-Giemsa, staining; B: Negative for peroxidase staining; C: Weak positive for periodic acid
Schiff staining.
Discussion
Recurrent translocations are often found in leukemia. The karyotype at diagnosis emerges as the most significant prognostic factor and provides the framework for current risk-stratified treatment approaches. The outcome of AML with the MLL rearrangements of chromosome band 11q23 with more than fifty distinct partner genes was considered to confer adverse prognosis. Therefore, AML with the MLL rearrangements are considered candidates for allogenetic transplant. t(4;11) (q21;q23) is one of the most frequent MLL rearrangements. Most leukemias with t(4;11) are classified as ALL, and 3% or so are AML, the majority AML-M4 and AML-M5 [12]. Now, we found one AML-M0 case with t(4;11)(q21;q23) by R-banding and M-FISH. What’s more important, we also observed a novel translocation t(7;11)(q36;p11) in this case which has never been reported before. The segment of 7q36 was too short to be identified, so we didn’t find the der(11)t(7;11) by M-FISH. The exact mechanism and clinical value of this translocation is worth further investigation. The first induction chemotherapy regimen of IA was ineffective, which revealed that this case had a very poor prognosis and suggested that t(4;11)(q21;q23) and t(7;11)(q36;p11) are adverserisk cytogentics. 21 months post-HSCT, he developed granulocytic sarcoma at diverse sites, which indicated that t(4;11)(q21;q23) and t(7;11)(q36;p11) may be predisposing factor of EMR. FLAG regimen was an effective treatment for this poor prognosis ANLL-M0 with t(4;11)(q21;q23) and t(7;11)(q36;p11).
Extramedullary relapse following allo-HSCT occur in up to 20% of patients with AML. Most of them are concurrently involved in bone marrow. Isolated extramedullary relapse of myeloid malignancies following allo-HSCT are rare [13-15]. In a retrospective survey of European transplantation centers, about 95% extramedullary relapses were followed by bone marrow relapses within 1-12 months after diagnosis. Only 0.65% of patients with AML undergoing allo- BMT developed isolated extramedullary recurrences [16]. The sites of extramedullary relapse are diverse, such as skin, the brain, head and neck, gastrointestinal tract, breast, spinal canal, testis, bone and pleura [17]. However, granulocytic sarcoma simultaneously presenting at diverse sites is very uncommon. This patient developed granulocytic sarcoma at testis, skin of legs, arms and abdomen, subcutaneous tissues of neck, eyelids and penis without concomitant bone marrow involvement for more than 18 months after extramedullary relapse diagnosis. The prognosis of extramedullary relapse is very poor, but prolonged survival has been observed in some patients. Extramedullary relapse post-HSCT should be treated in an individual way. The most effective management appears to be a combined systemic chemotherapy and local therapy, such as local excision and radiotherapy. DLI, which is an effective treatment in leukemia relapse after allo-HSCT [18,19], isn’t an effective treatment in controlling extramedullary relapse in CML or acute leukemia [20]. While, one study reported that successful use of DLI in a patient with isolated extramedullary relapse of ALL post bone marrow transplantation [21]. In the present case, the patient was treated using orchiectomy, radiotherapy, conventional chemotherapy and DLI at testis leukemia relapse and has remained disease-free status for 14 months. Furthermore, he showed rapidly tumor resolution of granulocytic sarcoma when he received a reduced intensive FLAG chemotherapy. The results demonstrated systemic chemotherapy in combination with local treatments were effective treatments for extramedullary relapse.
Conclusion
In conclusion, t(4;11)(q21;q23) and t(7;11)(q36;p11) are adverse risk of AML-M0. FLAG is a good choice for this kind of leukemia. Systemic chemotherapy in combination with local therapy is an effective approach for extramedullary relapse.
References
- Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985; 315: 550-554.
- Kakizuka A, Miller WH, Umesono K, Warrell RP, Fraukel SR, Murty VVVS, et al. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARa with a novel putative transcription factor, PML. Cell. 1991; 66: 663-674.
- Miyoshi H, Shimizu K, Kozu T, Masaki N, Kaneko Y, Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered with a limited region of a single gene, AML1. Proc Natl Acad Sci U S A. 1991; 88: 10431-10434.
- Tomblyn MB, Arora M, Baker KS, Blazar BR, Brunstein CG, Burns LJ, et al. Myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia: analysis of graft sources and long-term outcome. J Clin Oncol. 2009; 27: 3634-3641.
- Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016; 127: 62-70.
- Liu N, Ning HM, Hu LD, Jiang M, Xu C, Hu JW, et al. Outcome of myeloablative allogeneic peripheral blood hematopoietic stem cell transplantation for refractory/relapsed AML patients in NR status. Leuk Res. 2015; 39: 1375-1381.
- van Besien K. Allogeneic transplantation for AML and MDS: GVL versus GVHD and disease recurrence. Hematology Am Soc Hematol Educ Program. 2013; 2013: 56-62.
- Mo XD, Xu LP, Zhang XH, Liu DH, Wang Y, Chen H, et al. Chronic GVHD induced GVL effect after unmanipulated haploidentical hematopoietic SCT for AML and myelodysplastic syndrome. Bone Marrow Transplant. 2015; 50: 127-133.
- Copelan EA, Crilley PA, Szer J, Dodds AJ, Stevenson D, Phillips G, et al. Late mortality and relapse following BuCy2 and HLA-identical sibling marrow transplantation for chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2009; 15: 851-855.
- Rashidi A, Cashen AF. A cytogenetic model predicts relapse risk and survival in patients with acute myeloid leukemia undergoing hematopoietic stem cell transplantation in morphologic complete remission. Leuk Res. 2015; 39:77-81.
- Park J, Park SY, Cho HI, Lee D. Isolated extramedullary relapse in the pleural fluid of a patient with acute myeloid leukemia following allogeneic BMT. Bone Marrow Transplant. 2002; 30: 57-59.
- Zhao X, Li S, Li N, Fan R, Lin G, Wang X. 11q23 abnormalities in adult Chinese patients with hematological malignancies. Med Oncol. 2014; 31:115.
- Curley C, Durrant S, Kennedy GA. Is extramedullary relapse of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation associated with improved survival? Asia Pac J Clin Oncol. 2013; 9: 285-289.
- Harris AC, Kitko CL, Couriel DR, Braun TM, Choi SW, Magenau J, et al. Extramedullary relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Haematologica. 2013; 98: 179-184.
- Oshima K, Kanda Y, Yamashita T, Takahashi S, Mori T, Nakaseko C, et al. Central nervous system relapse of leukemia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2008; 14: 1100-1107.
- Bekassy AN, Hermans J, Gorin NC, Gratwohl A. Granulocytic sarcoma after allogeneic bone marrow transplantation: a retrospective European multicentre survey. Acute and chronic leukemia working parties of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1996; 17: 801-808.
- Cunningham I. Extramedullary sites of leukemia relapse after transplant. Leuk Lymphoma. 2006; 47: 1754-1767.
- Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukaemia in marrow transplant patients. Blood.1990; 76: 2462-2465.
- Levine JE, Barrett AJ, Zhang MJ, Arora M, Pulsipher MA, Bunin N, et al. Donor leukocyte infusions to treat hematologic malignancy relapse following allo-SCT in a pediatric population. Bone Marrow Transplant. 2008; 42: 201-205.
- Simpson DR, Nevill TJ, Shepherd JD, Fung HC, Horsman DE, Nantel SH, et al. High incidence of extramedullary relapse of AML after busulfan/cyclophosphamide conditioning and allogeneic stem cell transplantation. Bone Marrow Transplant. 1998; 22: 259-264.
- Lawson SE, Darbyshire PJ. Use of donor lymphocytes in extramedullary relapse of childhood acute lymphoblastic leukaemia following bone marrow transplantation. Bone Marrow Transplant.1998; 22: 829-830.