
Perspective
Ann Hematol Oncol. 2016; 3(10): 1119.
Discontinuation Outcomes Mutational Analysis BTK PLCγ2 Genes in CLL Patients Treated Ibrutinib
Sharma S*
Department of Hematology Oncology, West Los Angeles VA Medical Center, USA
*Corresponding author: Sanjai Sharma, Department of Hematology Oncology, West Los Angeles VA Medical Center, 11301 Wilshire Blvd Bldg. 500 Rm 4237 Los Angeles CA, 90073, USA
Received: September 28, 2016; Accepted: October 21, 2016; Published: October 24, 2016
Perspective
The highly effective Bruton tyrosine kinase (BTK) inhibitor, Ibrutinib is approved for the treatment of chronic lymphocytic leukemia (CLL) as initial therapy as well as relapsed disease.
Extensively pre-treated CLL patients demonstrate initial responses to Ibrutinib followed by drug resistance and disease progression in some cases. Disease progression on Ibrutinib can be progression of CLL, i.e., increasing leukemic cell counts or Richter transformation lymphoma (RT). Recent reports have analyzed probable causes of Ibrutinib resistance by sequencing BTK and PLCγ2 genes that allow interesting clinical correlation between disease progression and mutational analysis.
In the report from Ohio State [1] of a total of 308 patients who were on Ibrutinib, the cumulative discontinuation rate was 25% (76 out of 308 patients). Disease progression was observed in 31 out of 76 patients with rest discontinuing for other reasons. In the MD Anderson data of 127 patients, the discontinuation rate was 26% and the reason for discontinuation was disease progression (Richter transformation) in 7, CLL progression in 7, 3 patients went for stem cell transplantation, 2 patients discontinued for miscellaneous causes and 14 patients had adverse events or sudden death. Patients who discontinue Ibrutinib with a RT transformation have particularly poor prognosis with a median survival of only 3-4 months [2]. The outcome is slightly better in patients that have CLL progression on Ibrutinib [1,2]. Interestingly progression of CLL was seen later the course of Ibrutinib treatment than Richter’s transformation with a reported cumulative incidence of 4-6% while RT was observed earlier in the course of treatment with Ibrutinib with an incidence of 5-6% [1,2].
Maddocks, et al. [1] report sequencing data on BTK and PLCγ2 genes from CLL patients who progress on Ibrutinib. Both these genes are required for effective B-cell receptor signaling and mutations resulting in Ibrutinib resistance in patients have been previously described in both these genes [3]. The drug binds to the cysteine residue 481 (C481) and mutations have been reported that alter this residue as well as other residues of the BTK gene [3] (Figure 1). PLCγ2 gene mutations are gain of function mutations that also result in Ibrutinib resistance. The mutations in BTK and PLCγ2 genes are found in patients who experience a CLL progression (11 of 11 patients) on Ibrutinib and are in frequently seen in patients whose progression is a Richter transformation (RT, 2 of 9 patients) event (Figure 1). This appears logical as CLL progression is due to alteration of the target kinase, BTK so the drug is unable to block kinase activity and leukemic cells escape inhibition. In the case of Richter transformation, prior genomic analyses have reported that the resulting lymphoma is quite dissimilar to CLL at the genomic and transcriptional level [4]. Leukemic cells undergoing RT acquire a large number of additional novel driver events and signaling pathways that override the BTK inhibition which results in disease progression which is not necessarily associated with mutation in BTK gene itself. Maddocks, et al. report that in 2 of 9 Richter transformation patients BTK gene mutation was observed upon sequencing [1]. Interestingly these patients demonstrated a mixed pattern of progression with both RT and progressive CLL. It is plausible that in these cases the transformation is clonally related (BTK gene mutation present) to CLL in contrast to other RT transformations that are genetically distinct (clonally un-related, lack of BTK gene mutation).
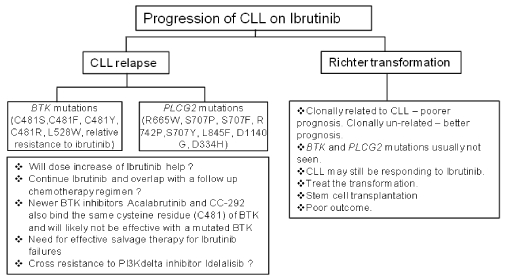
Figure 1: Schematic with possible outcomes of disease progression in CLL patients on Ibrutnib therapy and their relationship to BTK and PLCγ2 mutations.
With disease progression and mutations in BTK or PLCγ2, Ibrutinib may still be able to block some of its other known signaling targets (epidermal growth factor receptor (EGFR), tyrosine kinase expressed in hepatocellular carcinoma (TEC) and interleukin-2 inducible kinase (ITK) that have a role in CLL signaling pathways. Therefore an overlap strategy to continue this drug till clear responses are seen with an additional agent could be considered. Progression in the form of Richter transformation (RT) in a CLL patient on Ibrutinib portends a poor prognosis. This transformation can be clonally related or clonally distinct from CLL and in cases of clonally distinct RT, CLL may continue to respond to Ibrutinib but the real problem is the transformation that needs to be treated expeditiously. On the other hand there will not be any role of Ibrutinib in CLL patients with clonally related RT as they frequently will have BTK and PLCγ2 gene mutations.
Ibrutinib resistant leukemic cells will potentially continue to respond to other B-cell receptor pathway inhibitors that are downstream of BTK including Ras/Raf/MEK/ERK, PI3 kinase-Akt, NFKappa B pathways etc.Their efficacy may be lower than a BTK inhibitor as this kinase is involved in the very early steps of B-cell receptor signaling while the above mentioned pathways are active further downstream. It is unclear whether newer PI3Kd inhibitor, Idelalisib would be active in Ibrutinib resistant CLL leukemic cells and specifically in cells that are Ibrutinib resistant due to PLCγ2 gene mutations. PI3 Kinase functions upstream of PLCγ2 however both of them can be activated independently by B cell receptor signaling [5] and studies on B-cell receptor signaling have shown that inhibition of both PI3 Kinase and PLCγ2 activity is required to block this signaling activity. The clinical data on the efficacy of Idelalisib after Ibrutinib resistance is not available but based on other cellular models Idelalisib would have some growth inhibitory activity in these resistant leukemic cells.
Ibrutinib and other newer BTK inhibitors such as Acalabrutinib and CC-292 [6,7] are effective drugs for CLL as its blockade results in very high response rates. The role of gene mutations that result in drug resistance is therefore of great clinical relevance. At this time the sequencing analysis of BTK and PLCγ2 genes in CLL patients progressing on Ibrutinib is relevant to understand the biology and mechanism of resistance to BTK inhibitors. As all BTK inhibitors [6,7] are targeting the C481 site of the kinase, theoretically these inhibitors will demonstrate cross-resistance but this is currently not known. Identifying mutations in BTK and PLC2 genes at the time of CLL progression may become relevant if the BTK inhibitors that are currently under development have different activity profiles in the presence of specific BTK and PLCγ2 gene mutations.
References
- Maddocks KJ, Ruppert AS, Lozanski G, Heerema NA, ZhaoW, Abruzzo L, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients with Chronic Lymphocytic Leukemia. JAMA Oncol. 2015; 1: 80-87.
- Jain P, Keating M, Wierda W, Estrov Z, Ferrajoli A, Jain N, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015; 125: 2062-2067.
- Woyach JA, Furman RR, Liu TM Hatice Gulcin Ozer, Marc Zapatka, Amy S, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014; 370: 2286-2294.
- Fabbri G, Khiabanian H, Holmes AB, Wang J, Messina M, Mullighan CG, et al. Genetic lesions associated with chronic lymphocytic leukemia transformation to Richter syndrome. J Exp Med. 2013; 210: 2273-2288.
- Dai X, Chen Y, Schuman J, Hua Z, Adamson JW, Wen R, et al. Distinct roles of phosphoinositide-3 kinase and phospholipase Cgamma2 in B-cell receptor-mediated signal transduction. Mol Cell Biol. 2006; 1: 88-99.
- Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016; 374: 323-332.
- Brown JR, Harb WA, Hill BT, Gabrilove J, Sharman JP, Schreeder MT, et al. Phase 1 study of single-agent CC-292, a highly selective Bruton's tyrosine kinase inhibitor, in relapsed or refractory chronic lymphocytic leukemia. Haematologica. 2016; 101: e295-e298.