
Case Report
Ann Hematol Oncol. 2016; 3(11): 1121.
Fatal Rituximab-Induced Nonspecific Interstitial Pneumonia: Case Report and Review of the Literature
Sato R1, Molligan J2 and Gaballa S3*
1Department of Internal Medicine, Thomas Jefferson University, USA
2Department of Pathology, Thomas Jefferson University, USA
3Department of Medical Oncology, Thomas Jefferson University, USA
*Corresponding author: Sameh R. Gaballa, Department of Medical Oncology, Thomas Jefferson University, 925 Chestnut Street Suite 420A, Philadelphia, PA 19107, USA
Received: September 16, 2016; Accepted: October 29, 2016; Published: November 01, 2016
Abstract
Rituximab treatment can be associated with respiratory complications such as cough, bronchospasm, sinusitis, and rhinitis. Rarely, rituximab can cause fatal lung injury. We report the case of a patient on rituximab monotherapy who experienced shortness of breath and eventual respiratory failure despite steroid treatment. Limited biopsies of her bilateral lungs post-mortem revealed nonspecific interstitial pneumonia. We review the previous literature on rituximab-induced lung disease and use this case to highlight the need for close monitoring, early detection and treatment, especially for patients who have a previous history of lung disease.
Keywords: Interstitial lung disease; Nonspecific interstitial pneumonia; Rituximab
Abbreviations
COPD: Chronic Obstructive Pulmonary Disease; CT: Computed Tomography; BiPAP: Bilevel Positive Airway Pressure; ICU: Intensive Care Unit; NSIP: Nonspecific Interstitial Pneumonia; RTXILD: Rituximab-Induced Lung Disease; DLCO: Diffusing Capacity of the Lungs for Carbon Monoxide; NLRP3: Nod-like receptor pyrin domain-containing protein 3
Introduction
Rituximab is a chimeric anti-CD20 antibody that is used to treat some hematological malignancies such as B-cell lymphomas, and various autoimmune diseases including immune thrombocytopenic purpura, systemic lupus erythematous, rheumatoid arthritis, and autoimmune hemolytic anemia. Most common side effects include fever, chills, and rigors. Respiratory complications such as cough, bronchospasm, sinusitis, and rhinitis have also been reported in 30% of patients in clinical trials [1]. Rituximab-induced lung injury is a very rare but potentially fatal complication. The reported incidence ranges from 3.7 to 10%, and may even be higher as some cases may be regarded as lower respiratory tract infections. Of those with rituximab-induced lung injury it has been fatal in 18 to 30% of cases [2-5]. We report a case of fatal single agent rituximab-induced nonspecific interstitial pneumonia to increase awareness about this serious side effect and review the current literature.
Case Presentation
This case involves a 72-year-old female with stage IV marginalzone B-cell lymphoma who required therapy due to transfusiondependent anemia and was initiated on single agent rituximab (4 weekly doses at a dose of 375 mg/m2). She tolerated the first two infusions well, but started to feel short of breath after her third infusion. By the time she arrived to the infusion center for her fourth dose, she was short of breath at rest and was found to be hypoxic to 68% on room air. Of note the patient had COPD with a 30pack-year smoking history, quit 3 years ago, but had no oxygen requirements prior. She was admitted to the hospital where she was started on broad-spectrum antibiotics and placed on 5 liters of oxygen. A chest CT with contrast showed extensive bilateral ground-glass opacities with interlobular septal thickening (Figure 1A). On the second day of admission the patient became hypoxic to 70% on 6 liters but did not tolerate BiPAP so she was transferred to the medical ICU. Methylprednisolone 125 mg every 6 hours was started intravenously for possible rituximab-induced lung reaction on day 2. A repeat CT scan of the chest two weeks later revealed improvement in the bilateral airspace opacities (Figure 1B). However she clinically continued to deteriorate during this time and she eventually required intubation and mechanical ventilation. Bronchoscopy was performed which was negative for infection including any aerobic or anaerobic bacteria, acid-fast bacilli, fungus, or virus. The patient further decompensated with hypoxemic respiratory failure and expired on the third week of admission despite multiple rounds of resuscitation. The patient’s family agreed to an autopsy limited to a biopsy of her lungs. Two intercostal incisions measuring approximately 8cm in length were created bilaterally on the chest wall. The internal examination was limited due to the minimally invasive nature of the autopsy. On gross examination the pleural surface was smooth and glistening. There were no observable adhesions and the parenchyma was crepitant and firm. A limited biopsy of her bilateral lungs revealed a histologic pattern of nonspecific interstitial pneumonia with uniform thickening of the alveolar septa with a monotonous lymphoid infiltrate. There was spatial and temporal homogeneity seen (Figure 1C).
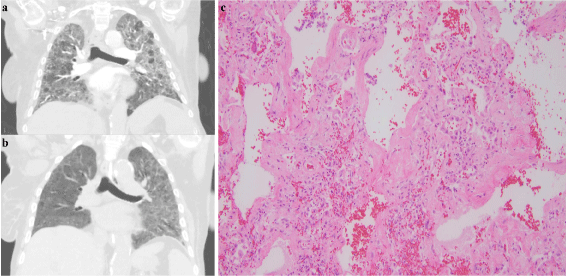
Figure 1: CT chest of this patient on admission (A) showed extensive ground glass opacification and septal thickening throughout the lungs bilaterally. After two
weeks of steroid therapy the CT chest showed improvement in the extensive bilateral airspace opacities (B). The biopsy of the autopsy lung showed histologic
features of uniform interstitial inflammation with varying degrees of fibrosis consistent with nonspecific interstitial pneumonia (C).
Discussion
Nonspecific interstitial pneumonia (NSIP) is a type of idiopathic interstitial pneumonia that is characterized by spatially and temporally uniform interstitial inflammation with varying degrees of fibrosis on pathology [6]. NSIP can be idiopathic but can be associated with the toxic effects of drugs and occupational exposure. Rituximab has been reported in the literature to be a cause of NSIP, as well as other noninfectious- related interstitial lung diseases [2,4,5,7]. The incidence of RTX-ILD was initially reported as 0.01 to 0.03%, however recent case series report ranges from 3.7 to 10% [2]. The complicating factor with diagnosis and prognosis of RTX-ILD is that rituximab is often used with concomitant chemotherapy or radiation which may also cause pulmonary toxicity, unlike our patient who only had rituximab monotherapy. Although several systematic reviews of the literature have noted RTX-ILD with rituximab monotherapy [2,4,5,8], only two reviews directly compared rituximab monotherapy to concomitant therapy. Blitzen, et al. noted in a review of pediatric cases that the clinical presentations and outcomes were similar in patients with and without concomitant chemotherapy [7]. Lioté, et al. noted in their systematic review that nine patients experienced no further respiratory symptoms when chemotherapy was restarted without rituximab, implying that rituximab was the culprit for lung injury [5].
The mechanism behind rituximab-induced lung disease (RTXILD) is complex and remains unclear, but is thought to be secondary to complement-dependent cell lysis [9], antibody dependent cellular toxicity, and tumor necrosis factor alpha release [1,3,4,10,11]. Rituximab efficiently eliminates B cells in lung tumors, normal lung tissues, and lung-associated lymph nodes, which may lead to normal lung injury through pro-inflammatory effects of activated cytokines, inflammatory mediators, and angiogenic factors [12,13]. There may also be interference of lymphocyte crosstalk due to prolonged B-cell depletion, causing cytotoxic T lymphocyte dysregulation, thereby promoting lung damage [2,14,15].
Our case report reveals the severe and potentially life-threatening pulmonary toxicity of rituximab. Similar to our patient, typical rituximab-induced lung injury is subacute in onset becoming symptomatic after a median of 4.1 doses [2]. The earliest presenting symptoms are often dry cough, exertional dyspnea, and fever. In 20% of cases patients are asymptomatic at the time of diagnosis with the disease being detected either by CT or pulmonary function tests [2]. Common radiologic findings on CT scan include ground glass opacification, alveolitis, pulmonary fibrosis, alveolar hemorrhage, pleural effusion and consolidation. Our patient did not get pulmonary function tests but they typically show a restrictive pattern on spirometry and significant DLCO reduction. Biopsy is not usually done but most commonly show pulmonary inflammation, and a range of histologic patterns can be seen including nonspecific interstitial pneumonia as seen in our patient [1].
The recommended treatment is prompt discontinuation of rituximab, initiation of steroids, and supportive treatment. However, there are no recommendations on dose, route, or duration of steroids [1-4,7,8]. In a number of cases such as ours, high doses of steroids were unable to prevent death [2]. Our patient received high-dose steroids on the second day, but despite improvement on imaging after steroid initiation our patient ultimately expired. Improvement on her imaging suggests the steroids were treating the NSIP, but the treatment could have been initiated too late or the injury to an already diseased lung could have been too devastating.
If the patient survives the rituximab-induced lung injury, it is unclear whether to avoid or reintroduce rituximab, as there have been cases of both positive and negative results [5]. As for other treatment options, inhibiting the complement pathway [9] or the activation of Nod-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome [13] have also been discussed in the literature but no cases have been reported thus far. Wu et al. reported a case of RTXILD treated with methyl prednisolone together with an antibody against tumor necrosis alpha therapy (Etanercept), but the benefits were only transient [3]. Further investigation into treatment options and guidelines for RTX-ILD is necessary.
As for how we could have prevented RTX-ILD in our patient, aside from educating the patient and close monitoring during treatment with rituximab with imaging and lung function tests, there are no recommendations on preventing RTX-ILD. Administration of glucocorticoids continuously or intermittently with each rituximab treatment cycle did not appear to prevent development of lung injury in pediatric patients or adult patients [2,7].
The risk factors for development of RTX-ILD remain unclear. Apart from Vulsteke, et al. who reported a case of acute exacerbation of idiopathic pulmonary fibrosis with rituximab use [16], most studies have looked at RTX-ILD in patients with no previous lung injury. Our patient had an extensive smoking history and COPD and the further damage that rituximab had on her lung proved to be fatal despite intensive steroid therapy. It is possible that RTX-ILD is more common in patients with pre-therapy pulmonary diseases such as COPD or an extensive smoking history, however, this needs to be confirmed in larger observational studies. Interestingly, Franzen, et al. identified cigarette smoking as a possible risk factor for pulmonary function decline with rituximab and recommended stopping during treatment [17].
In conclusion, lung injury such as nonspecific interstitial pneumonia is a rare but serious side effect of rituximab. Awareness and education of providers and patients, as well as close monitoring, early detection and treatment are key to preventing fatal incidents of rituximab-induced lung injury. Routine assessment of lung function by pulmonary function tests may be justified before and during treatment with rituximab, especially for patients who have a previous history of lung disease. New respiratory symptoms or radiologic changes should be taken seriously and investigations should include blood gases, a high-resolution CT of the chest, and pulmonary function tests. Suspicion for RTX-ILD should prompt discontinuation of the drug and a therapeutic trial of high-dose steroids, in order to prevent severe morbidity and mortality.
References
- Naqibullah M, Shaker SB, Bach KS, Bendstrup E. Rituximab-induced interstitial lung disease: five case reports. Eur Clin Respir J. 2015.
- Hadjinicolaou AV, Nisar MK, Parfrey H, Chilvers ER, Ostor AJ. Non-infectious pulmonary toxicity of rituximab: a systematic review. Rheumatology (Oxford) 2012; 51: 653-662.
- Wu Y, Jia Y, Xu J, Shuai X, Wu Y. Fatal interstitial lung disease induced by rituximab-containing chemotherapy, treatment with TNF-alpha antagonist and cytokine profiling: a case-report and review of the literature. J Clin Pharm Ther. 2013; 38: 249-253.
- Wagner SA, Mehta AC, Laber DA. Rituximab-induced interstitial lung disease. Am J Hematol. 2007; 82: 916-919.
- Lioté H, Lioté F, Seroussi B, Mayaud C, Cadranel J. Rituximab-induced lung disease: A systematic literature review. Eur Respir J. 2010; 35: 681-687.
- Kligerman SJ, Groshong S, Brown KK, Lynch DA. Nonspecific interstitial pneumonia: radiologic, clinical, and pathologic considerations. RadioGraphics 2009; 29: 73-87.
- Bitzan M, Anselmo M, Carpineta L. Rituximab (B-cell depleting antibody) associated lung injury (RALI): a pediatric case and systematic review of the literature. Pediatr Pulmonol. 2009; 44: 922-934.
- Zayen A, Rais H, Rifi H, Ouarda M, Afrit M, Cherif A, et al. Rituximab-induced interstitial lung disease: case report and literature review. Pharmacology. 2011; 87: 318-320.
- van der Kolk LE, Grillo-Lopez AJ, Baars JW, Hack CE, van Oers MH. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001; 115: 807-811.
- Bienvenu J, Chvetzoff R, Salles G, Balter C, Tilly H, Herbrecht R, et al. Tumor necrosis factor alpha release is a major biological event associated with rituximab treatment. Hematol J. 2001; 2: 378-384.
- Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003; 22: 7359-7368.
- Joly-Battaglini A, Hammarstrom C, Stankovic B, Aamodt H, Stjarne J, Brustugun OT, et al. Rituximab efficiently depletes B cells in lung tumors and normal lung tissue. F1000Res. 2016; 5: 38.
- Kong H, Wang Y, Zeng X, Zhu Q, Xie W, Dai S. Involvement of NLRP3 inflammasome in rituximab-induced interstitial lung disease: a case report. J Clin Pharm Ther. 2014; 39: 691-694.
- Alexandrescu DT, Dutcher JP, O’Boyle K, Albulak M, Oiseth S, Wiernik PH. Fatal intra-alveolar hemorrhage after rituximab in a patient with non-Hodgkin lymphoma. Leuk Lymphoma. 2004; 45: 2321-2325.
- Selenko N, Maidic O, Draxier S, Berer A, Jager U, Knapp W, et al. CD20 antibody (C2B8)-induced apoptosis of lymphoma cells promotes phagocytosis by dendritic cells and cross-priming of CD8+ cytotoxic T cells. Leukemia. 2001; 15: 1619-1626.
- Vulsteke C, Dierickx D, Verbeken E, Wolter P, Thomas J, Schoffski P. Rituximab-induced fatal interstitial pneumonitis: case report. Leuk Lymphoma. 2010; 51: 546-548.
- Franzen D, Ciurea A, Bratton DJ, Clarenbach CF, Latshang TD, Russi EW, et al. Effect of rituximab on pulmonary function in patients with rheumatoid arthritis. Pulm Pharmacol Ther. 2016; 37: 24-29.