
Special Article - Acute and Chronic Myeloid Leukemia
Ann Hematol Oncol. 2016; 3(11): 1124.
a-ß T/CD19-Depleted Haploidentical Hematopoietic Stem Cell Transplant (HSCT) as a Rescue Strategy in Blast-Phase Chronic Myeloid Leukemia Relapsing after a Matched Unrelated HSCT with Extramedullary Involvement: A Case Report
Cambò B1, Crugnola M1, Prezioso L1, Sammarelli G1, Bonomini S1, Craviotto L1, Mancini C2, Martella EM2, Masselli E1*, Bonifazi F3, Arpinati M3 and Aversa F1
1Department of Clinical and Experimental Medicine, Parma University Hospital, Italy
2Department of Diagnostics, Parma University Hospital, Italy
3Bone Marrow Transplant Unit, Bologna University Hospital, Italy
*Corresponding author: Elena Masselli, Hematology and BMT Unit, Department of Clinical and Experimental Medicine, Parma University Hospital, Via Gramsci n. 14, 43126 Parma, Italy
Received: November 02, 2016; Accepted: December 02, 2016; Published: December 05, 2016
Perspective
Blast crisis (BC) and extramedullary involvement (EI) are rare and aggressive clinical manifestations of chronic myelogenous leukemia (CML) that challenge the patient treatment algorithms also in the TKI-era. An adequate therapeutic approach for these patients need to consider the intrinsic biology of this disease, which is one of the most immunogenic hematologic neoplasm. Here we present an emblematic case of a patient with BC-CML, initially treated by allogenic bone marrow transplant from HLA-matched unrelated donor, who relapsed with EI under TKI maintenance. Only a-ß T cell depleted haploidentical stem cell transplant allowed to achieve stable disease remission, leading us to speculate that preserving the immunologic properties of the graft and avoiding immunosuppressive therapy is of utmost relevance in the transplantation strategy of CML.
Keywords: Chronic myelogenous leukemia; Blast crisis; Extramedullary involvement; Tyrosine kinase inhibitors; Hematopoietic stem cell transplant; TCR a-ßT-cell depletion
Introduction
Blast crisis (BC) and extramedullary involvement (EI) are both rare, aggressive clinical manifestations of chronic myelogenous leukemia (CML), that confer a dismal prognosis and challenge the conventional therapeutic approach to this disease. While EI by CML occur sporadically [1-4], up to 6.7% patients are in BC at the time of diagnosis.
Although the introduction of tyrosine kinase inhibitors (TKI) had a profound impact in altering the natural history of CML, reducing the disease progression rate from >20% to 1-1.5% per year, their effectiveness in uncommon presentations such as EI and BC is still controversial. In this setting, a therapeutic algorithm that considers allogenic hematopoietic stem cell transplantation (HSCT) configures as the only curative chance for these patients also in the TKI-era [5]. Here we present a case in which a a-ßT cell/CD19-depleted haploidentical HSCT successfully rescued a patient with BC-CML who had relapsed with EI after allogeneic transplant from a HLAmatched unrelated donor.
Case Presentation
In November 2009, a 42 year-old man was referred to our institution for leucocytosis (WBC= 28x109/L), moderate anemia (Hb =10.2 g/dl) and mild splenomegaly. The patient complained constitutional symptoms and presented a relevant history of previous exposure to chemotherapeutic agents for testicular lymphoma.
A diagnosis of blastic transformation of a chronic myeloproliferative disease was done on the basis of the peripheral blood morphology (Figure 1A), bone marrow aspirate immunophenotype (Figure 1B) and bone marrow biopsy showing increased cellularity with marked granulocytic precursor hyperplasia accompanied by reduced erythro- and megakaryopoiesis and a blast count of 40%. Cytogenetic analysis revealed the Philadelphia chromosome in the 56% of the examined metaphases and one metaphase with the following readout, encompassing all the so called “major route” abnormalities: 57, XY, t(9;22)(q34;q11), +der(22)t(9;22)(q34;q11.2), +6, +7, +8, +9, +10, +14, +19, +21, +21, +21 (Figure 2A and B). FISH analysis confirmed the presence of extra-copies of the Philadelphia chromosome and molecular analysis was positive for BCR-ABL hybrid gene (b3a2 p210).
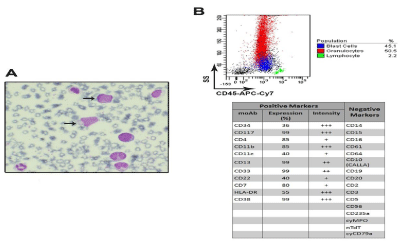
Figure 1: (A) Representative image of peripheral blood smear at the time of diagnosis showing left-shift leukocytosis with circulating blasts (arrows) (May Grunwald-
Giemsa staining, magnification 100X, Zeiss microscope). (B) Multicolor flow cytometric analysis of bone marrow aspirate at the time of diagnosis, showing ~45%
blasts (upper panels) expressing early (CD34, C117) and myeloid (CD13, CD33, CD11c, CD11b) antigens (lower panel). Aberrant expression of CD7 was
documented too. Samples were acquired on dual laser BD FACS-Canto II and analysis performed by FACSDiva software.
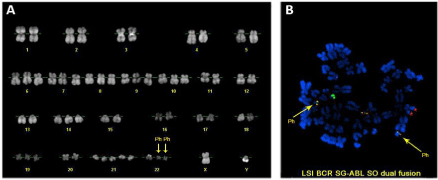
Figure 2: Cytogenetic analyses. (A) Karyogram showing hyperdiploid karyotype with duplication of Philadelphia chromosome; (B) Metaphase FISH with LSI BCR
SG ABL SO dual fusion probe showing the duplication of BCR-ABL fusion gene (yellow signal) on the two Philadelphia chromosomes and the third fusion signal of
the reciprocal ABL-BCR. Philadelphia chromosomes (Ph) are indicated by arrows.
Given the low response rate and poor progression free survival of TKI therapy alone in BP CML [6], the patient was managed with a combination of first generation TKI (Imatinib 400 mg daily then escalated up to 600 mg daily) and conventional AML-like induction chemotherapy (fludarabine-cytarabine-idarubicin, FLAI). A complete hematologic (CHR) and cytogenetic remission (CcyR), with BCR-ABL transcript detectable by nested-PCR (Figure 3) were obtained. Despite a maintenance therapy with Imatinib 600 mg daily as a bridge to allogeneic HSCT from unrelated donor, the disease relapsed after two months. At this point, mutational analysis was performed, revealing negative.
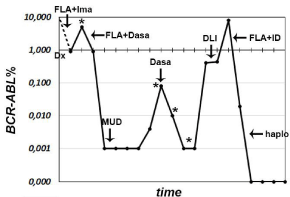
Figure 3: Peripheral blood BCR-ABL transcript monitoring by quantitative RTPCR
at different time points till the last follow up. Therapeutic interventions
are indicated by arrows (see text for details). FLA= fludarabine-cytarabineidarubicin,
Ima= imatinib, Dasa= Dasatinib, MUD= allogeneic HSCT from
10/10-matched unrelated donor, DLI= donor lymphocyte infusion, haplo=
haploidentical HSCT; *= Mutational analysis.
A second CCyR was achieved after a second course FLAI followed by Dasatinb (140 mg daily) for the next three months. In November 2010 (BCR-ABL= 0,001%, Figure 3) allogeneic HSCT from a HLA 10/10-matched unrelated donor was performed. Peripheral blood cells were the source of donor stem cells. Myeloablative conditioning regimen with busulfan (12.8 mg/Kg) and cyclophosphamide (120 mg/ Kg) was administered and GvHD prevention included anti-thymocyte globulin (ATG, 30 mg/Kg), short-term methotrexate (15 mg/m2 day +1, +3, +6, +11) and cyclosporine, the latter discontinued 6 months after HSCT. Subsequent follow up documented major molecular response (MMR) and full-donor chimerism until September 2011, when lost of MMR (BCR-ABL=0.10%, mutational analysis negative, see Figure 3) was controlled for one year by Dasatinib 140 mg daily, which allowed to achieve a transient Complete Molecular Response (Figure 3).
Definitive loss of molecular response (BCR-ABL=0.4%, mutational analysis negative, Figure 3) was documented in May 2012, and the patient was subsequently candidate to two donor lymphocyte infusions (DLI) (1x107 and 6x107 CD3+-cells/Kg, respectively, in timeframe of two months) associated to Dasatinib therapy (140 mg daily), with poor response (BCR-ABL=0.44% in December 2012, Figure 3).
In January 2013, the patient complained diffuse bone pain. A magnetic resonance documented several osteolytic lesions corresponding to multiple areas of augmented metabolic activity at the 18fludeoxyglucose positron-emission tomography-computed tomography (PET-CT) scan. Biopsy of the bone marrow and of a right scapular lesion showed robust infiltration by leukemic blasts (Figure 4A and B) whose karyotype presented the same cytogenetic abnormalities of that at time of diagnosis. A further salvage therapy with FLA and two courses of daunorubicin citrate liposome (lD) to boost the hematologic remission allowed patient to achieve CCyR and MMR (BCR-ABL=0,019%, Figure 3). PET-CT scan performed after lD revealed minimal residual bone disease. Twenty days after the last lD, a HSCT from one-haplotype mismatched sibling donor was performed. The donor was selected also on the basis of the donorversus- recipient Natural Killer (NK) cell alloreactivity. Conditioning regimen included ATG (6 mg/Kg), treosulfan (30 g/m2), thiotepa (10 mg/Kg) and fludarabine (150 mg/m2). Peripheral blood cells were the source of donor stem cells. Graft processing included a selective elimination of TCR a-ßT lymphocytes and CD19+ cells by CliniMACS® Separator (Miltenyi Biotech, Germany). The final inoculum consisted of a mega dose of CD34+ cells (to facilitate engraftment), a 4.5–5 log depleted T lymphocytes (to prevent GvHD), a huge numbers of NK, dendritic cells, monocytes and TCR γdT lymphocytes (to ensure antileukemic and anti-infective effects). Details of the graft composition are reported in Table 1. This specific graft manipulation allowed to avoiding immunosuppressive therapy.
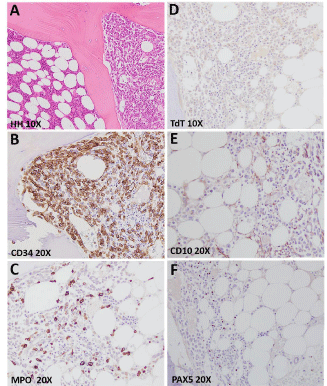
Figure 4: Bone marrow trephine biopsy at disease relapse after allo-HSCT
showing (A) lacunae with normal hematopoiesis (on the left) closed to lacunae
with massive infiltration by leukemic blasts (on the right) (hematoxylin-eosin
staining, magnification 10X, Olympus microscope); (B-F) Immunostaining
for CD34 and myeloperoxidase - MPO (positive), and CD10, TdT and PAX5
(negative), confirming the myeloid nature of the leukemic bone marrow
infiltrates (magnification 10-20X, Olympus microscope).
CD34+ cells
CD3 cells
CD20+ cells
NK cells
Monocytes
total CD3
TCRαβ
TCRγd
Total/kg
11x106
4.8x106
6x104
4.6x106
3.4x104
36x106
277x106
Table 1: Graft composition. Cell populations of the final inoculum (total/kg), showing a megadose of CD34 cells, a 4.5-5 log depleted TCRαβ T-lymphocytes and B-lymphocytes (CD20+ cells), and a huge numbers of NK, monocytes and TCR γdT-lymphocytes.
Both conditioning and early post-transplant phase were tolerated without significant toxic or infectious complications. Neutrophil (=0.5x109/L) and platelet (=20x109/L) recovery was documented at day +10 and +11 post HSCT, respectively. The patient experienced a skin-limited grade II acute GvHD that recovered after a short course of steroids and TKI.
Immunological reconstitution was rapid and effective, as documented by a number of CD4+ cells stably = 100/mm3 already at +1 months from transplant (Figure 5, panel A). This element, together with a similar rapid expansion of the other components of the adaptive and innate immunity (CD8+, Taß, Tγd, NK and B lymphocytes, see Figure 5 panels B-F) conferred an early and effective protection against infection. Indeed, at day +48, the patient experienced asymptomatic CMV reactivation that was cleared without any pharmacological treatment as we could track a peak in circulating CD8+ Taß, Tγd, NK cells (Figure 5 panels B-E) as well as the presence of CMV-specific CD8+ T lymphocytes by CMV dextrameric analysis (Figure 6).
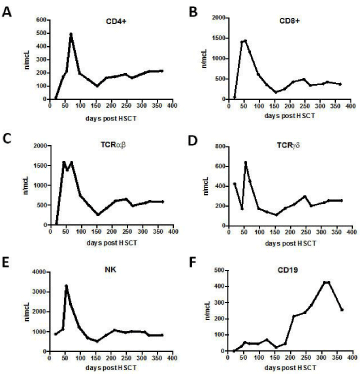
Figure 5: Lymphocyte subsets at day +50, +100, +150, +200, +250, +300,
+350, +400 post haplo-HSCT. The graphs show the number of: CD4+ cells/μl
(panel A), CD8+ cells/μl (panel B), TCRαβ cells/μl (panel C), TCRγd cells/μl
(panel D), NK cells/μl (panel C), B cells (CD19+)/μl.
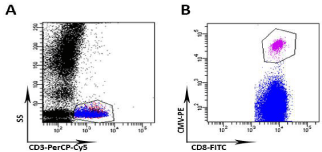
Figure 6: Flow cytometric quantification of CMV-specific CD8+ T cells by
Dextramer® CMV Kit (IMMUDEX Aps, Copenhagen). At day +68 post haplo-
HSCT, whole blood from the patient was stained with a PE-conjugated HLA
A02 (corresponding to donor’s HLA) Dextramer and labeled with anti-CD3
PerCP-Cy5.5 and anti-CD8-FITC using the general staining procedure. (A)
CD3-positive cells are identified by morphological gate (side scatter, SS)
and anti-CD3 PerCP-Cy5.5 staining (B) The gate identifies CMV A02 CD8-
positive T cells which represented the 1.3% on total lymphocytes (15 cells/
μl). Samples were acquired on dual laser BD FACS-Canto II and analysis
performed by FACSDiva software.
The patient is still in complete molecular response (Figure 3) with a full donor chimerism 3.5 years from HSCT.
Discussion/Conclusion
In the TKI-era, the possibility to achieve profound and stable molecular responses by oral drugs has somehow softened our notion of CML as a potentially lethal hematologic malignancy. However, losses of drug-response, clonal selection, and progression to advanced-phase disease are unfavourable events that need to be carefully considered and that ground their biological roots into TKI inactivity against CML stem cells [7-9].
CML is one of the most immunogenic cancer and, in fact, a strong immunologic control of the disease has a major role in determining the therapeutic efficacy of TKI, as well as in producing durable molecular remissions after immune-based therapies, i.e., HSCT and post-transplant DLI [10-12].
Our patient presented with an aggressive disease (as indicated by its clinical onset as BC, a karyotype displaying the so called “major route mutations” and a relapse with EI), poorly controlled by both conventional and target therapy, but successfully rescued by TCR aßT cells depleted haploidentical HSCT. The biological explanation of this relies into the immunological properties of this graft, which is depleted of the immune cells that mainly account for GvHD (TCR aßT cells), and enriched in a large number of effector cells (TCR γdT cells and NK cells). TCR γdT cells combine conventional adaptive features with direct, rapid responses against pathogens as well as a relevant anti-leukemic activity, as they directly recognized stressinduced self-antigens expressed by malignant cells [13]. Moreover is known that also NK cell alloreactivity, especially in haploidentical HSCT setting, has a major role on the transplant outcome via a strong GVL effect without increased risk of GVHD [14]. Furthermore, the myeloablative conditioning and the absence of any post-transplant immunosuppression may have contributed to eradicate the disease by debulking the disease before the infusion of the graft and by eliminating the residual leukemic stem cells via a GvL effect exerted by the T lymphocytes whose alloreactivity was not counteract by immunosuppressive agents. Finally, we should also take into account that lD, due to its affinity for reticular endothelium, contributed to destroy CML cells in the extra-hematologic sites.
This experience confirms the results we have so far achieved in adults with haematological malignancies using a HSCT strategy based on a selective depletion of the T lymphocytes from the G-CSFmobilized peripheral blood stem cells [15]. This approach guarantees a fast and sustained full-donor type engraftment, a prevention of both acute and chronic GvHD without the need of any additional post-transplant immunosuppression, a rapid and effective recovery of anti-infective and anti-leukemic immunity. Furthermore, in the absence of GvHD, this transplant procedure can be offer also to the end-stage patients and that the quality of life of the long-term survivors is extremely good.
Acknowledgement
We express our appreciation to the nursing staff of the Hematology and Bone Marrow Transplantation Unit of Parma University Hospital and to our charity association ParmAIL (Italian Association Against Leukemia, section of Parma).
References
- Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007; 21: 340-350.
- Specchia G, Palumbo G, Pastore D, Mininni D, Mestice A, Liso V. Extramedullary blast crisis in chronic myeloid leukemia. Leuk Res. 1996; 20: 905-908.
- Agis H, Weltermann A, Fonatsch C, Haas O, Mitterbauer G, Müllauer L, et al. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol. 2002; 81: 90-95.
- Soni A, Paluri R, Deal T, Peker D. Extramedullary Involvement by Chronic Myelogeneous Leukemia in Five Patients With Unusual Clinico-pathologic Features: A Review of the Effectiveness of Tyrosine Kinase Inhibitors. J Clin Med Res. 2016; 8: 480-485.
- Saußele S, Silver RT. Management of chronic myeloid leukemia in blast crisis. Ann Hematol. 2015; 94:159-165.
- Mukherjee S, Kalaycio M. Accelerated Phase CML: Outcomes in Newly Diagnosed vs. Progression From Chronic Phase. Curr Hematol Malig Rep. 2016;11: 86-93.
- Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107: 4532-4539.
- Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Humanchronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011; 121: 396-409.
- Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002; 99: 319-325.
- Zitvogel L, Rusakiewicz S, Routy B, Ayyoub M, Kroemer G. Immunological off-target effects of imatinib. Nat Rev Clin Oncol. 2016;13: 431-446.
- Ilander M, Kreutzman A, Mustjoki S. IFNa induces prolonged remissions modelling curative immunologic responses in chronic myeloid leukemia. Oncoimmunology. 2014; 3: e28781.
- Biernacki MA, Marina O, Zhang W, Liu F, Bruns I, Cai A, et al. Efficacious immune therapy in chronic myelogenous leukemia (CML) recognizes antigens that are expressed on CML progenitor cells. Cancer Res. 2010;70: 906-915.
- Aversa F. T-cell depletion: from positive selection to negative depletion in adult patients. Bone Marrow Transplant. 2015; 50: S11-113.
- Ruggeri L, Aversa F, Martelli MF, Velardi A. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol Rev. 2006; 214: 202-218.
- Martelli MF, Aversa F. Haploidentical transplants using ex vivo T-cell depletion. Semin Hematol. 2016; 53: 252-256.
Citation:Cambò B, Crugnola M, Prezioso L, Sammarelli G, Bonomini S, Craviotto L, et al. a-ß T/CD19-Depleted Haploidentical Hematopoietic Stem Cell Transplant (HSCT) as a Rescue Strategy in Blast-Phase Chronic Myeloid Leukemia Relapsing after a Matched Unrelated HSCT with Extramedullary Involvement: A Case Report. Ann Hematol Oncol. 2016; 3(11): 1124. ISSN : 2375-7965