
Case Report
Ann Hematol Oncol. 2016; 3(12): 1125.
Plasma Cell Leukemia Presenting with Spontaneous Tumor Lysis Syndrome: Report of a Rare Case and Literature Review
Yu Z¹*, Ogunleye F¹, Blankenship LM¹, Ward N², Ezekwudo DE¹, Stender M¹, Gaikazian SS¹, Huang JZ² and Jaiyesimi I¹
¹Department of Hematology and Oncology, Oakland University William Beaumont School of Medicine, USA
²Department of Pathology and Laboratory Medicine, Oakland University William Beaumont School of Medicine, USA
*Corresponding author: Zhou Yu, Department of Hematology and Oncology, William Beaumont Hospital, 3577 W. 13 Mile Rd., Suite 202a, Royal Oak, MI, USA
Received: November 07, 2016; Accepted: December 27, 2016; Published: December 30, 2016
Abstract
Plasma cell leukemia (PCL) is a rare but aggressive plasma cell neoplasm. It is defined by more than 20% plasma cells in the peripheral blood or an absolute plasma cell count of more than 2x109/L. We describe an interesting case of PCL in a 46-year-old African American female who presented with rib pain, fatigue, anemia, acute renal failure, leukocytosis with 85% plasma cells and spontaneous tumor lysis syndrome (TLS). TLS was managed with intravenous hydration and antihyperuricemic therapy. Despite an initial favorable response after induction chemotherapy, the patient’s disease progressed and she expired from infection and multi-organ failure eight months later. A detailed report of this case and a review of the management of TLS, plasma cell leukemia diagnosis and treatments are presented here.
Keywords: Plasma cell leukemia; Renal failure; Tumor lysis syndrome
Abbreviations
PCL: Plasma Cell Leukemia; TLS: Tumor Lysis Syndrome; MM: Multiple Myeloma; ORR: Overall Response Rate; OS: Overall Survival; ASCT: Autologous Hematopoietic Cell Transplantation
Introduction
Plasma cell leukemia (PCL) is a rare but aggressive lymphoproliferative disorder with a poor prognosis. It accounts for 2-3% of all plasma cell neoplasms. PCL is defined by more than 20% plasma cells in the peripheral blood or an absolute plasma cell count of more than 2x109/L [1]. PCL usually has a more aggressive clinical presentation than multiple myeloma (MM), including a higher incidence of diffuse bone marrow involvement, hepatomegaly, splenomegaly, lymphadenopathy, leptomeningeal infiltration, and extramedullary soft tissue plasmacytomas [1]. Here we report a case of PCL whose clinical course represented the rare and aggressive aspects of this disease.
Case Presentation
A 46-year-old African American female presented with two months history of worsening right-sided rib pain, shortness of breath and fatigue. Her past medical history included hypertension and a four pack-year smoking history. Family history was unremarkable for solid or hematological malignancies. Examination revealed pallor, chest wall tenderness, tachycardia and bilateral lower extremity edema. Her initial laboratory results showed anemia, marked leukocytosis, thrombocytopenia, azotemia, hyperuricemia and hypercalcemia. Urinalysis showed 2+ proteinuria and amorphous crystals. Table 1 summarizes the relevant laboratory findings.
Laboratory tests
2 months prior to admission
On admission
Reference range
Hemoglobin(g/dL)
14.1
7.3
12-15
Platelet count(cells/mm3)
116,000
17,000
150,000 – 400,000
Total WBC(cells/mm3)
5,200
125,200
4,000-10,000
Serum creatinine (mg/dL)
0.66
2.78
0.6-1.4
Serum BUN(mg/dL)
12
47
8-22
Potassium (mEq/L)
4.2
4.2
3.5-5.2
Phosphorus(mg/dL)
NA
5.9
2.3-4.3
Uric acid(mg/dL)
NA
12.5
1.5-6.0
Calcium (mg/dL)
9.2
13.6
8.4-10.4
β2-microglobulin(mg/L)
NA
25.95
0.00-1.9
NA: Not Available
Table 1: Laboratory values at hospital admission.
With hyperuricemia, hyperphosphatemia and acute kidney failure, tumor lysis syndrome (TLS) was highly suspected. Immediately the patient was given intravenous normal saline for aggressive hydration, forced diuresis with furosemide and one dose of rasburicase (6 mg IV) for hyperuricemia. Calcitonin and one dose of pamidronate were also administered to treat hypercalcemia.
The peripheral blood smear revealed leukocytosis with lymphocyte-like plasma cells (Figure 1). Flow cytometry analysis of peripheral blood showed 85% plasma cells that were positive for CD138, CD38, dim positive for CD45, negative for CD19 and CD56, and with kappa light chain restriction (Figure 2). A diagnosis of PCL was made. We failed to obtain bone marrow aspiration as specimen was a dry tap. The bone marrow core biopsy confirmed the diagnosis of PCL with massive (90%) bone marrow involvement. Serum protein electrophoresis did not show significant M-band. The serum free kappa light chain level was elevated at 338 mg/dL (normal 0.35-2.49 mg/dL) with an increased free kappa/lambda ratio of 751 (normal 0.27 to 1.80).A skeletal survey revealed numerous lytic lesions.
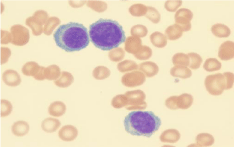
Figure 1: Peripheral blood smear showing plasma cells with eccentric nuclei,
perinuclear halos with basophilic cytoplasm.
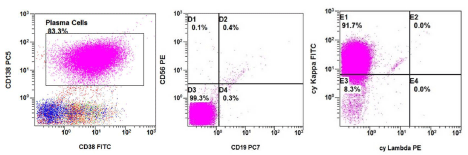
Figure 2: Flow cytometry analysis of peripheral blood.
The patient’s hyperuricemia, hyperphosphatemia and hypercalcemia improved significantly with initial treatments (Figure 3A). Chemotherapy was initiated on day 4 with systemic bortezomib, cyclophosphamide and dexamethasone. Leukocytosis resolved by day 15. The patient’s creatinine level had decreased initially with TLS treatments and then with initiation of chemotherapy the creatinine gradually rose as well as uric acid level (Figure 3B). Allopurinol 200 mg daily was started to decrease uric acid formation. Postrenal obstruction was ruled out by image studies. Renal biopsy was contraindicated due to a high bleeding risk from thrombocytopenia. Given the strikingly high free light chain level, the likelihood of light chain nephropathy was great. Plasmapheresis was initiated on day 7 and renal function significantly improved after five sessions without the need for hemodialysis (Figure 3B).
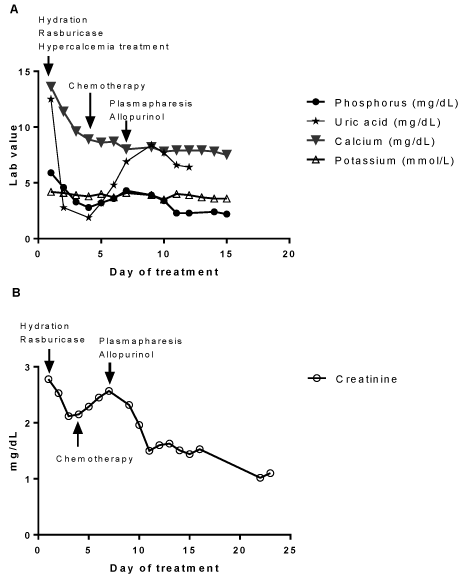
Figure 3: Changes of laboratory parameters in response to treatments.
After four cycles of chemotherapy, her free light chain kappa/ lambda ratio completely normalized and a repeat bone marrow biopsy showed 5-8% plasma cell involvement. According to International Myeloma Work Group response criteria, she had achieved partial remission [1]. Unfortunately, she later developed complications including pathological fracture which delayed the planned autologous bone marrow transplantation. Her disease progressed and she expired from infection and multi-organ failure eight months after her diagnosis.
Discussion
PCL is a pathologic entity distinct from multiple myeloma with aggressive features and a poor prognosis. Here we described a case of PCL with the unusual presentation of spontaneous TLS.
TLS is an oncologic emergency comprised of metabolic disturbances resulting from either spontaneous or cytotoxic therapyinduced tumor cell death. The lysis of a large number of tumor cells releases intracellular contents, including nucleic acids, proteins, and electrolytes, into the systemic circulation which leads to the development of hyperuricemia, hyperphosphatemia, hyperkalemia and secondary hypocalcemia. This may cause multi-organ dysfunction including acute renal failure, cardiac arrhythmia and seizure [2]. TLS is most often seen after initiation of chemotherapy. In the absence of therapy, spontaneous TLS can occur, although it is rare, mostly in patients with acute leukemia and highly aggressive non-Hodgkin’s lymphoma. According to the Cairo and Bishop Classification system, in the absence of cytotoxic therapy or other triggering factors, this patient met the criteria for clinical TLS as she presented with laboratory derangements of hyperuricemia, hyperphosphatemia and acute renal failure [1]. To our knowledge, this is the first report of spontaneous TLS occurring in PCL.
TLS is extremely rare in plasma cell neoplasms due to the low proliferation rate of plasma cells. However, in the setting of extremely high tumor burden, TLS can occur. TLS has been reported in MM patients who were treated with bortezomib [3-9], carfilzomib [10,11], thalidomide [7,12-15], corticosteroidalone [16], or spontaneously [17]. TLS occurrence in PCL has been described only in one case after initiation of bortezomib treatment [18]. The definition of high tumor burden which potentially increases risks of TLS in plasma neoplasm is not clear in the literature. It is believed to be correlated with increased β-2 microglobulin, elevated lactate dehydrogenase, high percentage bone marrow involvement, immature morphology and advanced bone lytic lesions. It has been proposed that light chain nephropathy may be associated with a higher risk of TLS, a scenario similar to our patient [15]. PCL is the most aggressive form of plasma cell neoplasm with a high tumor burden and a high prevalence of organ involvement at presentation [19]. Our patient’s characteristics reflects the aggressive nature of this disease, represented by her extreme peripheral plasmacytosis, advanced bone lytic lesions and massive bone marrow infiltration, all of which predisposed her to a high risk of TLS.
Acute renal failure, as seen in our patient, was a sequela of multiple factors, including uric acid and calcium phosphate crystallization, superimposed upon light chain nephropathy. Adequate hydration, diuresis as well as reducing uric acid level with the use of rasburicase particularly are essential to preserve or improve renal function [2]. Hyperphosphatemia can be less remarkable in spontaneous TLS than in chemotherapy-induced TLS, and presumably results in a lesser degree of secondary hypocalcemia [20]. This phenomenon may be due to actively growing malignant cells rapidly take up extracellular phosphate released from dying cells, causing a neutral net phosphate flux [20]. The hypercalcemia observed here may represent a net outcome between PCL causing hypercalcemia and TLS causing secondary hypocalcaemia. Hypercalcemia and normocalcemia in TLS have been previously reported [17,21]. Although evidence for utilizing plasmapheresis in light chain nephropathy is limited to uncontrolled retrospective case series, it is a reasonable therapy for individuals with extremely high free light chain level (above 200 mg/ dL).
Due to the rarity of PCL, there have been no large prospective randomized trials of treatments. Recommendations are largely based on case reports, small retrospective series and data extrapolated from the treatment of MM. Historically, outcomes in PCL are dismal, with a median survival of 6 to 12 months [22]. This early mortality is likely due to the typical initial aggressive presentation with complications as well as the lack of effective therapy to achieve sustained response.
series and retrospective studies reported that regimens containing bortezomib, a proteasome inhibitor, had better disease control with an overall response rate (ORR) ranging from 69% to 89.9% and an overall survival (OS) ranging from 12-24 months [22- 25]. Lenalidomide, an immunomodulatory drug, showed an ORR of 74% and a median OS of 28 months in a very small phase II prospective trial [26]. Several retrospective studies of high-dose chemotherapy followed by autologous hematopoietic cell transplantation (ASCT) demonstrated prolonged OS and ASCT may be of value, although no definite conclusion can be drawn due to the lack of randomized prospective studies [19,27,28].
Conclusion
Our report raises the need for clinical awareness of spontaneous TLS in PCL. Although uncommonly encountered, TLS should not be overlooked as it is associated with significant mortality. Laboratory parameters need to be closely monitored. Improvement in PCL outcomes requires focusing on two aspects: reducing early mortality and achieving sustained long term disease control. Early recognition of PCL and management of its complications are essential first steps. Induction chemotherapy should be initiated immediately to achieve rapid cytoreduction and minimize the risks contributing to early death. Strategies to improve long term survival include incorporating novel therapies with induction, consolidation and post-graft maintenance treatment stages. Prospective randomized trials investigating these approaches are necessary.
References
- Fernández de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DH, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013; 27: 780-791.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011; 364:1844-1854.
- Terpos E, Politou M, Rahemtulla A. Tumour lysis syndrome in multiple myeloma after bortezomib (VELCADE) administration. J Cancer Res Clin Oncol. 2004;130: 623-625.
- Kenealy MK, Prince HM, Hönemann D. Tumor lysis syndrome early after treatment with bortezomib for multiple myeloma. Pharmacotherapy. 2006; 26:1205-1206.
- Sezer O, Vesole DH, Singhal S, Richardson P, Stadtmauer E, Jakob C, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006; 7: 233-235.
- Arai A, Oshikawa G, Kurosu T, Miki T, Tohda S, Koyama T, et al. [Bortezomib-induced tumor lysis syndrome with a remarkable elevation of LDH in a case of relapsed and therapy-resistant multiple myeloma]. Rinsho Ketsueki. 2006; 47: 777-780.
- Chim CS. Rapid complete remission in multiple myeloma with bortezomib/thalidomide/dexamethasone combination therapy following development of tumor lysis syndrome. Cancer Chemother Pharmacol. 2008; 62: 181-182.
- Dhanraj KM, Biswajit D. Tumor lysis syndrome in multiple myeloma treated with bortezomib. J Pharmacol Pharmacother. 2014; 5: 161-162.
- Wang L, Jian Y, Yang G, Gao W, Wu Y, Zuo L. Management of tumor lysis syndrome in patients with multiple myeloma during bortezomib treatment. Clin J Oncol Nurs. 2015; 19: E4-7.
- Shely RN, Ratliff PD. Carfilzomib-associated tumor lysis syndrome. Pharmacotherapy. 2014; 34: e34-37.
- Howard SC, Trifilio S, Gregory TK, Baxter N, McBride A. Tumor lysis syndrome in the era of novel and targeted agents in patients with hematologic malignancies: a systematic review. Ann Hematol. 2016; 95: 563-573.
- Cany L, Fitoussi O, Boiron JM, Marit G. Tumor lysis syndrome at the beginning of thalidomide therapy for multiple myeloma. J Clin Oncol. 2002; 20: 2212.
- Fuente N, Mae JM, Barcelo R, Muoz A, Perez-Hoyos T, Lopez-Vivanco G. Tumor lysis syndrome in a multiple myeloma treated with thalidomide. Ann Oncol. 2004;15: 537.
- Huston A, Brown J, Roodman GD. Tumor lysis syndrome following thalidomide and dexamethasone therapy for newly diagnosed multiple myeloma. Exp Hematol. 2006; 34:1616.
- Chang H, Lee SY, Tang TC. Tumor lysis syndrome in patients with light chain multiple myeloma: report of two cases. Chang Gung Med J. 2011; 34: 70-75.
- van de Kerkhof JJ, Peters WG, Visser J, Creemers GJ. Acute tumor lysis syndrome in a patient with multiple myeloma treated with dexamethasone monotherapy. Neth J Med. 2001; 59: 83-85.
- Saravu K, Kumar S, Shastry AB, Kurien A, Prabhu R, Kumar R. Spontaneous tumour lysis syndrome in a case of multiple myeloma - A rare occurrence. Australas Med J. 2013; 6: 168-1671.
- Jaskiewicz AD, Herrington JD, Wong L. Tumor lysis syndrome after bortezomib therapy for plasma cell leukemia. Pharmacotherapy. 2005; 25: 1820-1825.
- Tiedemann RE, Gonzalez-Paz N, Kyle RA, Santana-Davila R, Price-Troska T, Van Wier SA, et al. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008; 22: 1044-1052.
- Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014; 21:18-26.
- Shah BK. Hypercalcemia in tumor lysis syndrome. Indian J Hematol Blood Transfus. 2014; 30: 88-89.
- Katodritou E, Terpos E, Kelaidi C, Kotsopoulou M, Delimpasi S, Kyrtsonis MC, et al. Treatment with bortezomib-based regimens improves overall response and predicts for survival in patients with primary or secondary plasma cell leukemia: Analysis of the Greek myeloma study group. Am J Hematol. 2014; 89: 145-150.
- Pagano L, Valentini CG, De Stefano V, Venditti A, Visani G, Petrucci MT, et al. Primary plasma cell leukemia: a retrospective multicenter study of 73 patients. Ann Oncol. 2011; 22: 1628-1635.
- D'Arena G, Valentini CG, Pietrantuono G, Guariglia R, Martorelli MC, Mansueto G, et al. Frontline chemotherapy with bortezomib-containing combinations improves response rate and survival in primary plasma cell leukemia: a retrospective study from GIMEMA Multiple Myeloma Working Party. Ann Oncol. 2012; 23:1499-1502.
- Musto P, Rossini F, Gay F, Pitini V, Guglielmelli T, D'Arena G, et al. Efficacy and safety of bortezomib in patients with plasma cell leukemia. Cancer. 2007;109: 2285-2290.
- Musto P, Simeon V, Martorelli MC, Petrucci MT, Cascavilla N, Di Raimondo F, et al. Lenalidomide and low-dose dexamethasone for newly diagnosed primary plasma cell leukemia. Leukemia. 2014; 28: 222-225.
- Mahindra A, Kalaycio ME, Vela-Ojeda J, Vesole DH, Zhang MJ, Li P, et al. Hematopoietic cell transplantation for primary plasma cell leukemia: results from the Center for International Blood and Marrow Transplant Research. Leukemia. 2012; 26:1091-1097.
- Drake MB, Iacobelli S, van Biezen A, Morris C, Apperley JF, Niederwieser D, et al. Primary plasma cell leukemia and autologous stem cell transplantation. Haematologica. 2010; 95: 804-809.