
Case Report
Ann Hematol Oncol. 2017; 4(2): 1133.
A Case Report of Renal Artery Thrombosis in a Patient with Occult Neuroendocrine Tumor
Otoupalova E¹*, Blankenship L¹, Bazzi J¹, Ardary CW³, Nguyen T4, Momin F² and KelekarAK¹
¹Deptartment of Internal Medicine, Oakland University William Beaumont School of Medicine, USA
²Deptartment of Hematology and Oncology, Oakland University William Beaumont School of Medicine, USA
³Deptartment of Radiology, Oakland University William Beaumont School of Medicine, USA
4Deptartment of Pathology and Laboratory Medicine, Oakland University William Beaumont School of Medicine, USA
*Corresponding author: Otoupalova E, Department of Internal Medicine, William Beaumont Hospital, 2282 Dorchester Dr North Apt 206, Troy, 48084 Michigan, USA
Received: January 04, 2017; Accepted: February 13, 2017; Published: February 15, 2017
Abstract
Acute renal artery thrombosis is a rare diagnosis. In absence of underlying cardiac and hypercoagulable etiologies, underlying malignancy must be considered. We present a unique case and review of literature of renal artery thrombosis that led to discovery of an underlying neuroendocrine tumor. Thrombosis was likely provoked by underlying malignancy as well as circulating catecholamines. Patient was successfully treated with anticoagulation and somatostatin analogue.
Keywords: Renal artery thrombosis; Arterial thrombosis; Neuroendocrine tumor
Abbreviations
LDH : Lactate Dehydrogenase; CDX: Caudal type homeobox 2; CK7: Cytokeratin 7; TTF-1: Thyroid Transcription Factor-1; PAX-8: Paired-box gene 8
Introduction
Acute renal artery thrombosis is a rare diagnosis. In absence of other explanations, underlying malignancy must be considered. We present an unusual case of a patient that presented with renal artery thrombosis and subsequently was diagnosed with neuroendocrine tumor.
Case Presentation
The patient is a 58-year-old previously healthy female that presented to the emergency department for acute onset of left-sided flank pain. Her pain started several hours prior to presentation, radiated to her left upper quadrant and epigastric region and was associated with nausea. Review of systems was negative for fevers, chills, or weight loss.
Upon physical exam, her vital signs were normal. She had tenderness to deep palpation in the left upper quadrant and epigastric region. Initial laboratory workup revealed a leukocytosis of 12.9 bil/L. Basic metabolic panel, including creatinine, was unremarkable and urinalysis was normal. Lactate dehydrogenase (LDH) was slightly elevated at 258 U/L.
A computed tomography of abdomen revealed a well marginated, discrete lack of nephrogram involving a significant portion of the lower pole of the left kidney (Figure 1). Imaging also showed multiple hypodense and isodense lesions scattered throughout the liver parenchyma. An abdominal duplex revealed lack of flow to the left kidney in the segmental arteries of the mid pole and the arcuate/ interlobular arteries in the cortex of the mid and lower lobes. These findings were consistent with infarct of the left renal artery.
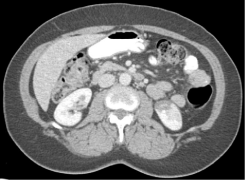
Figure 1: Radiographic evidence of left renal artery infarct.
Hospital course
The patient was admitted and unfractionated heparin IV was initiated. Hypercoagulable workup revealed normal anti-thrombin, protein C activity, antiphospholipid antibodies, anti-nuclear antibody and anti-neutrophilic cytoplasmic antibody and a mildly elevated protein S activity at 134% [normal 57-125%]. Carbohydrate antigen 19-9 was also elevated at 51.4 units/ml [0.0–34.9 units/ml]. Other tumor markers, including alpha-fetoprotein, cancer antigen 125 and carcinoembryonic antigen were normal. Echocardiography showed an ejection fraction of 55% with no evidence of thrombus formation. She maintained normal sinus rhythm throughout hospitalization. Liver magnetic resonance imaging was performed and revealed multiple hepatic lesions throughout the right and left hepatic lobes.
Patient underwent biopsy of the liver lesions that revealed neuroendocrine tumor grade 1 with extensive tumor necrosis. Immunohistochemistry was positive for anti-pan cytokeratin (AE1/ AE3), chromogranin and synaptophysin and strongly positive for caudal type homeobox 2 (CDX-2) highly suggestive of metastatic neuroendocrine tumor of lower intestinal tract. Ki-67 was positive in less than two percent of the tumor cells. Cytokeratin 7 (CK7), gastrin, thyroid transcription factor-1 (TTF-1) and paired-box gene 8 (PAX- 8) were negative (Figure 2, 3 and 4).The patient was transitioned to low molecular weight heparin with lovenox 1.5 mg/kg/day along with aspirin 81 mg daily.
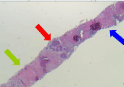
Figure 2: 2x H&E: Core needle biopsy of liver showing nests of neuroendocrine
tumor cells adjacent to uninvolved liver parenchyma and necrosis (arrows
correspond to text).
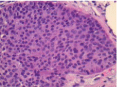
Figure 3: 40x H&E: Uniform, small, bland tumor cells in solid to trabecular
pattern. Well differentiated (low grade) tumor with monotonous, low mitotic
activity.
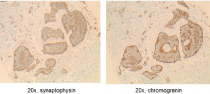
Figure 4: Tumor cells positive cytoplasmic staining by synaptophysin and
chromogranin, consistent with neuroendocrine tumor.
Further course
Patient was further evaluated for secretory polypeptides. Catecholamine panel showed only mild increase in dopamine (25 pg/ ml [0-20 pg/ml]) and norepinephrine (765 pg/ml [80-520 pg/ml]), and normal epinephrine (19 pg/ml [10-200 pg/ml]), free metanephrine (<0.20 nmol/L [<0.50 nmol/L]), and free normetanephrine (0.7 nmol/l [<0.90 nmol/L]). Chromogranin A was significantly elevated at 222 ng/ml [0-95 ng/ml] as well as serotonin at 2550 ng/ml [< 230 ng/ml] and urine 5-HIAA at 30.2 mg/24hr [2.0-6.0 mg/24hr].
Esophagogastrodudodenoscopy and colonoscopy were performed without evidence of malignancy. Octreotide scan showed increased uptake in the liver without other foci of abnormal uptake. CT enterography revealed multiple hyper-enhancing lesions identified within the small bowel, predominately the ileal loops of the small bowel (Figure 5). The diagnosis was determined to be metastatic neuroendocrine tumor of small bowel origin. Due to the high tumor burden within the liver and the multifocal disease of the small bowel, the patient was deemed not to be a surgical candidate. She was continued on lovenox SC and started on systemic therapy with a somatostatin analoglanreotide.
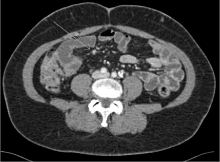
Figure 5: CT enterography with small bowel nodule.
After six months of therapy, the patient had repeat imaging with CT enterography revealing subtle liver lesions and only one focal area of small bowel wall enhancement in the jejunum measuring four milimeters. These findings as well as improvement in chromogranin A (108 ng/ml [0–95 ng/ml]) and serotonin (498 ng/ml [< 230 ng/ ml]) are consistent with a good response to somatostatin analog therapy. Repeat hypercoaguable work up was negative including anti-thrombin, protein C and S activity, antiphospholipid antibodies, prothrombin genotyping, and factor V leiden genotyping.
Discussion and Literature Review
Renal artery thrombosis
Renal infarction is an uncommon condition. The prevalence of renal infarction has been estimated at fourteen per 1000 persons [1]. Patients with acute renal infarction typically present with acute onset of flank pain or generalized abdominal pain, nausea, vomiting, and fever. Laboratory workup usually documents elevated LDH as well as decreased renal function. Hematuria is present in about one half of patients [2]. CT with contrast showing a wedge-shaped perfusion defect is diagnostic with 80 percent sensitivity. A radioisotope scan can be used if CT is non-diagnostic [3].
In the largest study evaluating causes of renal artery thrombosis by Bourgault, et al. [4], 94 patients were evaluated for etiology of renal infarction. The most common etiologies were renal infarction of cardiac origin and renal infarction associated with renal artery injury. Renal infarction associated with hypercoagulability disorders was least frequent and encompassed a total of fifteen patients with hereditary thrombophilia, hyperhomocysteinemia, antiphospholipid syndrome and nephrotic syndrome secondary to immunoglobulin light chain amyloidosis. Bolderman, et al. [5] studied 27 patients with apparent idiopathic renal artery thrombosis; sixteen patients of which did not have any detectable cardiac abnormality. In those patients, a high incidence (50%) of heritable thrombophilia and/or hyperhomocysteinemia as possible contributing factors was noted. Due to high overall incidence of hypercoagulable markers in general population, the causative role in arterial thromboembolic disorders remains controversial.
Role of neuroendocrine tumors in thrombosis
Cancer generates a prothrombotic state through the release of procoagulants, such as tissue factor and cancer procoagulant. Large population-based studies have shown that cancer patients have a 6-7-fold increased risk of developing venous thromboembolism compared with those without cancer [6]. The association with arterial thrombosis is less well described.
A large retrospective study by Amer, et al. [7] looked at 1,874 cancer patients of which 307 developed thrombosis during their life time. These patients included 230 (12.3%) with venous thrombosis, 28 (1.5%) cases with arterial occlusion, and 12 (0.6%) with combined venous and arterial thrombosis. Between patients with arterial thrombosis, the most prevalent was cerebrovascular thrombosis leading to stroke, myocardial ischemia with anginal symptoms, celiac artery occlusion with ischemic colitis and peripheral arterial occlusion with impending limb gangrene. Another study by El Sakka, et al. [8] evaluated 192 patients presenting with critical limb ischemia. In the study, 22 patients (11.5%) were found to have an underlying cancer. There was a wide range of malignancies, with most prevalent being lung cancer, hematological malignancy, gastrointestinal and hepatobiliary malignancies.
The role of neuroendocrine tumors in thrombosis development is unclear. Association of pheochromocytoma with arterial thrombosis, venous thrombosis and intracardiac thrombi has been well described in several case reports [9-17]. The etiology is attributed to several factors including endothelial damage from high blood pressure, mechanic compression of veins and release of procoagulant agents. Two case reports describe pheochromocytoma complicated by renal artery thrombosis [16,17]. The authors hypothesize that high levels of circulating catecholamines could have contributed to a prothrombotic state and vasospasms leading to renal artery thrombosis. Other types of neuroendocrine tumors associated with thrombosis are extremely rare. Upon review of literature we found three cases describing pancreatic NET associated with mesenteric thrombosis [18-20].
Our case is unique as it is a case of serotonin producing NET associated with thrombosis. Given rarity of both the type of tumor and renal artery thrombosis, it is likely that the two pathologies are related. We hypothesize that high serotonin level, together with catecholamines, might have contributed to prothrombotic state.
No studies have been conducted on length of anticoagulation in arterial thrombosis in cancer patients. Our patient responded well to treatment, however given concern for continuous procoagulant state, we decided to treat patient indefinitely or until malignancy is resolved.
Conclusion
We present an unusual case of renal artery thrombosis that led to discovery of a metastatic neuroendocrine tumor in our patient. It is likely that both presence of malignancy, circulating catecholamines and serotonin contributed to a prothrombotic state. As our case demonstrates, it is important to consider occult malignancy in patients with arterial thrombosis.
References
- Hoxie HJ, Coggin CB. Renal Infarction: Statistical study of two hundred and five cases and detailed report of an unusual case. Arch Intern Med. 1940; 65: 587-594.
- Domanovits H, Paulis M, Nikfardjam M, Meron G, Kürkciyan I, Bankier AA, et al. Acute renal infarction. Clinical characteristics of 17 patients. Medicine (Baltimore). 1999; 78: 386-394.
- Hazanov N, Somin M, Attali M, Beilinson N, Thaler M, Mouallem M, et al. Acute renal embolism. Forty-four cases of renal infarction in patients with atrial fibrillation. Medicine (Baltimore) . 2004; 83: 292-299.
- Bourgault M, Grimbert P, Verret C, Pourrat J, Herody M, Halimi JM, et al. Acute renal infarction: a case series. Clin J Am Soc Nephrol. 2013; 8: 392-398.
- Bolderman R, Oyen R, Verrijcken A, Knockaert D, Vanderschueren S. Idiopathic renal infarction. Am J Med. 2006; 119: 356.e9-12.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005; 293: 715-722.
- Amer MH. Cancer-associated thrombosis: clinical presentation and survival. Cancer Manag Res. 2013; 5: 165-178.
- El Sakka K, Gambhir RP, Halawa M, Chong P, Rashid H. Association of malignant disease with critical leg ischaemia. Br J Surg. 2005; 92: 1498-1501.
- Zhou W, Ding SF. Concurrent pheochromocytoma, ventricular tachycardia, left ventricular thrombus, and systemic embolization. Intern Med, 2009; 48: 1015-1019.
- Hou R, Leathersich AM, Ruud BT. Pheochromocytoma presenting with arterial and intracardiac thrombus in a 47-year-old woman: a case report. J Med Case Rep. 2001; 5: 310.
- Brauchlin AE, Rudiger A, Bächli EB, Schmid C, Maggiorini M. Portal vein thrombosis and liver failure in a patient with pheochromocytoma crisis. Am J Emerg Med. 2009; 27: 630.e3-630.e5.
- Kota SK, Jammula S, Meher LK, Modi KD. Pheochromocytoma with inferior vena cava thrombosis: An unusual association. J Cardiovasc Dis Res. 2012; 3: 160-164.
- Stevenson S, Ramani V, Nasim A. Extra-adrenal pheochromocytoma: an unusual case of deep venous thrombosis. J Vasc Surgery. 2005;42: 570-572.
- Robert B, Chivot C, Degremont R, Trouillet N, Lafaye-Boucher N, Gondry-Jouet C, et al. Thrombosis of the superior mesenteric vein revealing an ectopic pheochromocytoma of the organ of Zuckerkandl. Diagn Interv Imaging. 2012; 93: 625-628.
- Shigemura K, Tanaka K, Arakawa S, Hara I, Kawabata G, Fujisawa M. Malignant pheochromocytoma with IVC thrombus. Int Urol Nephrol. 2007; 39:103-106.
- Thewjitcharoen Y, Atikankul T, Sunthornyothin S. Renal infarction associated with adrenal pheochromocytoma. Urology. 2013; 82: e17.
- Lam J, Henríquez R, Cruzat C. Pheochromocytoma and von Recklinghausen neurofibromatosis: postpartum crisis and renal artery thrombosis. Rev Med Chil. 1998; 126: 1367-1367.
- Naswa N, Kumar R, Bal C, Malhotra A. Vascular thrombosis as a cause of abdominal pain in a patient with neuroendocrine carcinoma of pancreas: Findings on (68)Ga-DOTANOC PET/CT. Indian J Nucl Med. 2012; 27: 35-37.
- Nguyen BD. Pancreatic neuroendocrine tumor with portal vein tumor thrombus: PET demonstration. ClinNucl Med. 2005; 30: 628-629.
- Kawakami H, Kuwatani M, Hirano S, Kondo S, Nakanishi Y, Itoh T, et al. Pancreatic endocrine tumors with intraductal growth into the main pancreatic duct and tumor thrombus within the portal vein: a case report and review of the literature. Intern Med. 2007; 46: 273-277.