
Case Report
Ann Hematol Oncol. 2017; 4(2): 1137.
The Basophil Dilemma
Sidhwa K¹, Mansukhani D¹, Padate B², Gupta S² and Khodaiji S¹*
¹Department of Hematopathology, Laboratory Medicine, Hinduja National Hospital & Medical Research Centre, India
²Department of Hematology, Hemato-oncology and BMT, Hinduja National Hospital & Medical Research Centre, India
*Corresponding author: Khodaiji S, Department of Hematopathology, Laboratory Medicine, P.D, Hinduja National Hospital & Medical Research Centre, Veer Savarkar Marg, Mahim, Mumbai - 400016, India
Received: January 09, 2017; Accepted: February 25, 2017; Published: February 28, 2017
Abstract
Acute Myeloid Leukemia (AML) with basophilic differentiation i.e., Acute Basophilic Leukemia (ABL) and Chronic Myeloid Leukemia (CML) in basophilic blast crisis are two entities which are characterized by the primary differentiation into basophilic lineage as ascertained by electron microscopy, special stains and/or immunophenotyping. We report a case where we were faced with a dilemma of differentiating between these two entities on morphology.
The patient was a 60 year oldwoman who showed an excess of blasts with basophilic differentiation on peripheral blood and bone marrow at presentation. There was no previous history of a hematological malignancy. The Philadelphia (Ph) chromosome was positive on fluorescence in-situ hybridization (FISH). However Ph chromosome positivity has also been noted in ABL cases, further adding to the difficulty in diagnosis.
Keywords: Acute myeloid leukemia; Chronic myeloid leukemia; Philadelphia chromosome; Acute basophilic leukemia; Basophilic blast; Immunophenotyping
Introduction
Acute basophilic leukemia and basophilic blast crisis in CML are very rare diseases with only few reported cases. Differentiating between these two conditions can be very challenging due to overlapping morphology and immunophenotypic features. Further, established protocols or guidelines to distinguish these two entities are not readily available in literature. This distinction however is imperativein order tomake therapeutic decisions. Incidence of Ph positive AML appears to be very low, apparently constituting less than 1% of allnewly diagnosed cases of AML [1,2]. In a report of 8 cases of ABL by Peterson, et al. 3 were Ph chromosome positive [t(9;22)], without showing clinical features of CML, while no other chromosomal abnormality was found in the rest, including t(6;9) which is the abnormality commonly seen in AML patients with basophilia [3].
Generally, presence of Phchromosome,provides strong evidence for blast crisis arising in the background of CML [4]. However, Peterson, et al. have observed thatPh chromosome positivity can also be seen in ABL cases, thus adding to the difficulty in diagnosis [5]. We report a case illustrative of a diagnostic dilemma of ABL versus a CML in basophilic blast crisis.
Case Presentation
A 60-year-old female presented with a short, 10 days history of loss of appetite and generalized weakness. Physical examination of the patient revealed a massive splenomegaly. A complete hemogram showed a WBC of 136X109/L, Hemoglobin of 93 g/L and a platelet count of 155X109/L.
The peripheral blood smear showed a differential count comprising 9% blasts, 1% promyelocyte, 12% myelocytes, 9% metamyelocytes, 16% neutrophils, 3% lymphocytes, 6% monocytes, 41% basophils and 3% eosinophils. The blasts were large, showing basophilic cytoplasm, high N:C ratio and open chromatin. In addition a population of cells, interim in size between blasts and mature basophils was seen. These cells showed presence of few basophilic granules and were difficult to identify as they had overlapping features between basophilic blasts and basophilic promyelocytes/ myelocytes (Figure 1).
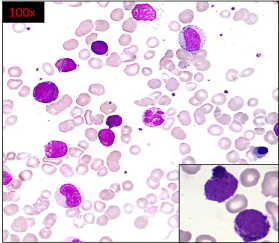
Figure 1: Leishman stained peripheral blood smear showing myeloid blasts
with basophilic granules along with myelocytes, metamyelocytes and mature
basophils was seen. Cells with overlapping features between basophilic
blasts and basophilic promyelocytes / myelocytes also seen.
A bone marrow examination was performed. The bone marrow aspirate showed a hypercellular marrow, with myeloid prominence and 14% blasts with similar morphology as seen in peripheral blood smear along with 26% mature and immature basophils (Figure 2). The distinctive feature noted in both the peripheral blood and bone marrow examination was the presence of cells with overlapping morphology as described earlier. Due to difficulty in classifying these cells, they were included in the basophil count.
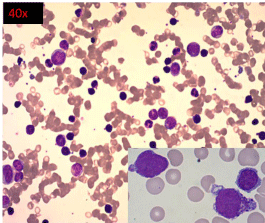
Figure 2: High power view (40X) of Leishman stained bone marrow aspirate
smear showing hypercellular marrow, with 14% blasts (inset).
Flow cytometric immunophenotyping done on bone marrow showed 42% cells in the blast region on CD45 vs side scatter (SSC) with expression of CD38, CD117, CD13 and CD33 antigens, suggesting an acute myeloid leukemia. A few blasts expressed CD34, while HLADR was dim to negative (Figure 3). In view of the morphology of blasts with basophilic granules, and immunophenotypic features of HLADR negativity in myeloid blasts, additional markerswere evaluated, of which CD123 and CD9 were positive [6], suggestive of basophilic differentiation [7,8], while CD25 was negative. Few cases in literature report expression of CD4 in basophilic blasts [9], which was also seen in our case.
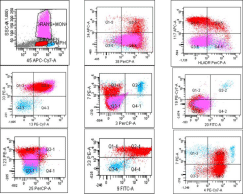
Figure 3: Scatter plots of flow cytometric immunophenotyping of bone marrow
showing expression of CD38, CD117, CD13 and CD33 antigens on blasts.
The bone marrow biopsy comprised diffuse sheets of immature cells, some with large nuclei, paucity of mature myeloid elements and decreased erythroid precursors (Figure 4). Megakaryocytes were seen.
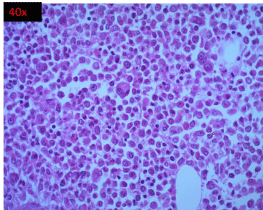
Figure 4: High power view (40 X) of H and E stained section of bone marrow
biopsy showing diffuse sheets of immature cells, some with large nuclei,
reduced mature myeloid elements and erythroid precursors and adequate
megakaryocytes.
Keeping the overall peripheral blood and bone marrow morphology and the flow cytometry findings in mind, the differential diagnoses considered were AML with basophilic differentiation arising de novo, versus a basophilic blast crisis in CML.
The BCR–ABL1 fusion gene was positive by Fluorescent In Situ Hybridization (FISH), following which the patient was started on Imatinib treatment but did not show a good response. Imatinib resistance mutation analysis (IRMA) showed sensitivity to Dasatinib. Therefore the drug was changed to Dasatinib, and her blood counts returned to normal.
Conventional cytogenetics studies were not available at the time of starting therapy. After 6 months of treatment, bone marrow examination showed no excess of blasts and only 2 percent basophils were seen with no evidence of BCR-ABL1 fusion t(9;22) by FISH.
Molecular workup for acute leukemia done later after starting therapy showed no evidence of DEK/NUP214: t(6;9), trisomy 8, trisomy 21 and TP53 deletion by FISH.
Discussion
In our case, the morphological findings on peripheral blood and bone marrow examination favored two possibilities; CML in blast crisis showing basophilic differentiation, or a de novo ABL. Our initial impression was a de novo ABL, because of increased myeloid blasts with basophilic differentiation as seen on morphology and flow cytometry. A primary presentation with a short history and without any prior history of CML in this patient further strengthened our diagnosis.
Earlier electron microscopy [7,10] and staining with toluidine blue were tools to identify basophilic blasts but with advent of basophil specific markers on flow cytometry, this may not be mandatory for diagnosis.
However, FISH for Philadelphia chromosome was positive, opening up the possibility of this being a case of CML in basophilic blast crisis.
Since the patient had splenomegaly and the Ph chromosome was positive by FISH, we decided to treat the patient with a TKI. Furthermore, the patient had poor cardiac function with 32% ejection fraction, deranged liver function along with pleural effusion, which prevented us from instituting chemotherapy for AML upfront.
As these cases are rare, very few case series and sporadic case reports are found in literature with hardly any consensus on diagnosis and treatment of this entity
One such case series of 8 cases of ABL by Peterson, et al. [5] has shown that ABL patients can present as de novo acute leukemia with a positive Ph chromosome. This was the case in our patient as well and therefore supported our initial impression.
In a case reported by Athena Kritharis, et al. [11], the author states that on flow cytometric immunophenotyping, these blasts with basophilic differentiation may expressCD9, CD45, CD13, CD33, CD123 and CD11b antigens. All these markers were expressed in our case.
In an attempt to address this complex issue, the 2016 revision of the World Health Organization classification of myeloid neoplasms and acute leukemia has introduceda new provisional category of AML with BCR-ABL1[12]. This new category attempted to recognize these rare de novo AML cases that may benefit from TKI therapy [13]. Although the diagnostic distinction between de novo AML with BCR-ABL1 and blast transformation of CML may be difficult without adequate clinical information, the significance of detecting this fusion as a provisional disease category is justified because appropriate targeted therapy may benefit these patients.
Preliminary data suggest that additional molecular markers such as deletion of antigen receptor genes (IGH, TCR), IKZF1 and/or CDKN2A may support a diagnosis of de novo ABL over blast crisis of CML [14]. However the category of AML NOS Acute basophilic leukemia, described in WHO 2008 remains unchanged in the revised WHO 2016 [12].
As per the WHO classification 2016, our case could be classified either as AML with BCR-ABL1 positive or AML NOS acute basophilic leukemia. Our dilemma persists and can only be dispelled with further molecular testing of the above markers.
To sum up, since our patient responded to a TKI (Dasatinib), showed presence of Ph chromosome, absence of other chromosomal aberrations, presented with massive splenomegaly and had adequate megakaryocytes on bone marrow examination the overall features favored diagnosis of CML in basophilic blast crisis over ABL.
Conclusion
The clinical findings, bone marrow examination, FISH and flow cytometry support the diagnosis of a very rare presentation of a not so rare disorder. The definite diagnosis of this case was difficult based exclusively on morphological findings. Flow cytometry, cytogenetic and molecular studies are mandatory in a patient presenting de novo with such florid findings as the diagnosis cannot be ascertained without them.
References
- Keung YK, Beaty M, Powell BL, Molnar I, Buss D, Pettenati M, et al. Philadelphia chromosome positive myelodysplastic syndrome and acute myeloid leukemia: retrospective study and review of literature. Leuk Res. 2004; 28: 579-586.
- Paietta E, Racevskis J, Bennett JM, Neuberg D, Cassileth PA, Rowe JM, et al. Biologic heterogeneity in Philadelphia chromosome–positive acute leukemia with myeloid morphology: the Eastern Cooperative Oncology Group experience. Leukemia. 1998; 12: 1881-1885.
- Peterson LC, Parkin JL, Arthur DC, Brunning RD. Acute Basophilic Leukemia: A Clinical, Morphologic, and Cytogenetic Study of Eight Cases. Am J ClinPathol. 1991; 96: 160-170.
- Gill RM, Etzell JE. Basophilic leukemia in recurrent chronic myelogenous leukemia blast phase. Am J Hematol. 2007; 82: 736-737.
- Peterson LC, Parkin JL, Arthur DC, Brunning RD. Acute basophilic leukemia: A clinical, morphologic, and cytogenetic study of eight cases. Am J Clin Pathol. 1991: 96; 160-170.
- Han X, Jorgensen JL, Brahmandam A, Schlette E, Huh YO, Shi Y, et al. Immunophenotypic study of basophils by multiparameter flow cytometry. Arch Pathol Lab Med. 2008; 132: 813-819.
- Arber DA, Brunning RD, Orazi A, Porwit A. Acute myeloid leukaemia, not otherwise specified. In:, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008 : 130-139.
- Babiker HM, Proytcheva M. Basophilic blast phase of chronic myelogenous leukemia. Blood. 2014; 124: 2464.
- Teshima T, Kondo S, Harada M, Shibuya T, Okamura T, Tamari Y, et al. Characterization of leukaemic basophil progenitors from chronic myelogenous leukaemia. Br J Haematol. 1991; 78: 55-59.
- Shvidel L, Shaft D, Stark B, Shtalrid M, Berrebi A, Resnitzky P. Acute basophilic leukaemia: eight unsuspected new cases diagnosed by electron microscopy. Br J Haematol. 2003; 120: 774-781.
- Kritharis A, Brody J, Koduru P, Teichberg S, Allen SL. Acute Basophilic Leukemia Associated With Loss of Gene ETV6 and Protean Complications. J Clin Oncol. 2011; 29: e623-e626.
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. Review Series- The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127: 2391-2405.
- Soupir CP, Vergilio JA, Dal Cin P, Muzikansky A, Kantarjian H, Jones D, et al. Philadelphia chromosome-positive acute myeloid leukemia: a rare aggressive leukemia with clinicopathologic features distinct from chronic myeloid leukemia in myeloid blast crisis. Am J Clin Pathol. 2007; 127: 642- 650.
- Nacheva EP, Grace CD, Brazma D, Gancheva K, Howard-Reeves J, Rai L, et al. Does BCR/ABL1 positive acute myeloid leukaemia exist? Br J Haematol. 2013; 161: 541-550.